Site Initiation Visit (SIV) Presentation: Clinical Trials
advertisement
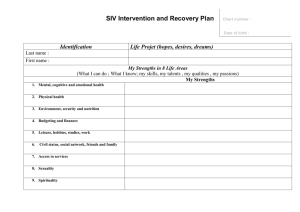
MODULE G INITIATION VISIT Jane Fendl April 14, 2010 Version: Final 14-Apr-2010 1 AGENDA Purpose of the visit Preparing for the visit Conducting the visit Source documents Contact information Writing the report Follow-up procedures & action items Version: Final 14-Apr-2010 2 Purpose of an Site Initiation Visit (SIV) To review/train the Investigator and staff on the protocol To review/train the Investigator and staff on all required protocol procedures To review the roles and responsibilities of all involved parties To confirm that the Investigator, facilities and staff are prepared to begin the study Communicate sponsor standards and expectations in regards to GCP Final step before conducting the actual study Version: Final 14-Apr-2010 3 Preparing for the SIV Review and confirm that all regulatory documents are acceptable and in the sponsor file Schedule a date with the site to perform the SIV ◦ Call to determine a date when the entire staff can be present Investigator, Sub-Investigators, Research fellows, Specialists, Study coordinator, Study nurse, Pharmacist, Laboratory Tech, Administrator Inform the Project Manager of date Version: Final 14-Apr-2010 4 Preparing for the SIV Send a confirmation letter with agenda to the Investigator A few days before call the site to: ◦ Confirm that the site has received all documents, study supplies ◦ Confirm that all administrative and logistical arrangements are completed Version: Final 14-Apr-2010 5 Preparing for the SIV Gather documents and materials for the visit Protocol and slide presentation Samples of drug packaging Samples of laboratory kits Blank CRF ◦ Annotated CRF ◦ CRF completion guidelines Logs Ship any necessary documents or materials so that they arrive at the site on time Version: Final 14-Apr-2010 6 Conducting the Visit Be prepared to document the visit by taking notes or using SIV report form Introduce your self, other sponsor staff and have site staff introduce themselves Attendance – have all the attendees sign a training form Be flexible with agenda – ask about staff schedules and adjust if necessary Version: Final 14-Apr-2010 7 Conducting the Visit See separate SIV presentation slides Version: Final 14-Apr-2010 8 Source Documents The first place that study documentation is recorded ◦ Lab reports, x-rays, ECG tracings, Progress notes, electronic records Review the Source Documents with the Study Coordinator ◦ Complete the Source Document log Version: Final 14-Apr-2010 9 Individual Contacts Meet with anyone who could not be present at the SIV meeting Inspect facilities to assure no changes occurred from the PSSV to the SIV Inspect the Clinical Drug Supplies ◦ ◦ ◦ ◦ Complete Damage Receipt documentation is present Blinding preserved Meet with the Investigator to review any outstanding issues and state if enrollment can begin Version: Final 14-Apr-2010 10 Writing the Report Refer to SIV Report Template Handout Version: Final 14-Apr-2010 11 Follow up Procedures & Action Items Complete visit by writing a “Follow-up Visit” letter to the Investigator Items to include: ◦ Thank the Investigator and staff for their attendance ◦ Confirm they can begin enrolling if applicable ◦ List outstanding items ◦ Offer resolutions to outstanding items ◦ Provide a possible date of next visit Version: Final 14-Apr-2010 12