Acids and Alkalis: Year 7 Chemistry Lesson Plan
advertisement
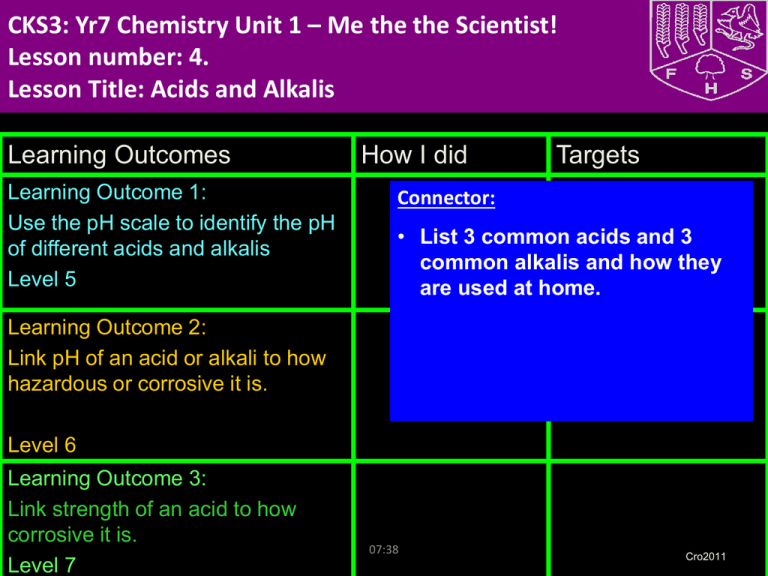
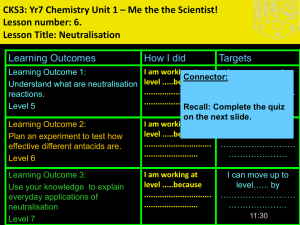
CKS3: Yr7 Chemistry Unit 1 – Me the the Scientist! Lesson number: 4. Lesson Title: Acids and Alkalis Learning Outcomes Learning Outcome 1: Use the pH scale to identify the pH of different acids and alkalis Level 5 How I did Targets Connector: • List 3 common acids and 3 common alkalis and how they are used at home. Learning Outcome 2: Link pH of an acid or alkali to how hazardous or corrosive it is. Level 6 Learning Outcome 3: Link strength of an acid to how corrosive it is. Level 7 07:38 Cro2011 New Information for Task 1 • Acids and alkalis are groups of chemicals. ALKALI ALKALI ACID ACID NEUTRAL ACID ALKALI Acids and alkalis When a substance dissolves in water it makes a solution. Solutions can be sorted by whether they are: acid, alkali or neutral. http://www.bbc.co.uk/learningzone/clips/universal-indicator-acid-alkalior-neutral/121.html New for Task 1alkalis ALKALIS SomeACIDS factsInformation about acids and Sharp, sour taste Soapy feel Strong acids can corrode (eat away) metals, e.g. hydrochloric acid Strong alkalis can corrode metals, e.g. sodium hydroxide Some can burn living tissue, e.g. battery acid, other kinds won’t, e.g. lemon juice Can be caustic (burn living tissue), e.g. caustic soda for oven cleaning Some are hazardous, e.g. sulphuric acid, some are harmless, e.g. vinegar Some are hazardous, e.g. sodium hydroxide, some are harmless, e.g. sodium bicarbonate (baking powder) Neutralise (cancel out) alkalis Neutralise (cancel out) acids Turn litmus indicator RED Turn litmus indicator BLUE Have a pH of 1-6 Have a pH of 8-14 Examples Examples hydrochloric acid sulphuric acid nitric acid citric acid (lemons, oranges etc) vinegar soap (Johnson’s pH 5.5) Sodium hydroxide (caustic soda) ammonium hydroxide (ammonia) calcium hydroxide (limewater) Washing powder Oven cleaner soap BIG picture Key Question: How can you determine if a chemical is an acid or an alkali? What skills will you be developing this lesson? HSW- by planning and carrying out an investigation/ Interpreting data/ evaluating an experiment ICT- through using laptops Numeracy- by using formulae in calculations Literacy- by writing explanations using correctly spelt keywords and good grammar. Team work- during a practical investigation Self management- by completing an individual assignment by ….. Creative thinking- by designing a ………………. Independent enquiry- by researching the internet Participation- during a practical activity Reflection- through self and peer assessment of each outcome 07:38 How is this lesson relevant to every day life? There are many acids and alkalis present in our everyday lives. Quick Discussion: What do you already know? Keywords: Create sentences using the keywords to show that you know what they mean. Put your hand up if there is any key word from the list that you don’t understand. • Acid • Alkali • Solution • pH • • • • • • Indicator • Universal Indicator • Sour taste • Soapy feel • Caustic 07:38 Irritant Strong Weak Corrode hazardous New Information for Learning Outcome 1 • Visual: Demonstration • http://www.bbc.co.uk/learningzone/clips/universal-indicatoracid-alkali-or-neutral/121.html • Audio: Demonstration • Kinaesthetic: Class experiment 07:38 What is the pH scale? The strength of an acid or alkali is measured by the pH scale. Each universal indicator colour is given a number called the pH value. Universal indicator can tell you the pH of a solution. stronger acid stronger alkali 1 2 3 4 5 6 7 8 9 10 11 12 13 14 strong acid weak weak acid neutral alkali What is the pH of a weak acid? What is the pH of strong alkali? strong alkali Copy into books Task 1 (Level 5) • Task 1: • Predict from the range of household substances which ones you think are acids and alkalis, write them down in predictions • Task 1: Extension • Group the substances into strong and weak acids and strong and weak alkalis Keywords for Task 1: • Acid • Alkali • Weak Acid • Weak Alkali • pH • Universal indicator • Copy and complete Substance Milk Apple juice Orange juice Lemonade Cola Vinegar Bleach Water Prediction, acid or alkali? Colour and pH Acid or alkali? What type of substance? Are these substances acidic or alkaline? Are they weak or strong? Substance soda water car battery acid 1 Description of acid/alkali very weak acid very strong acid soap 8 very weak alkali washing soda 10 weak alkali stomach acid 2 strong acid oven cleaner 14 very strong alkali vinegar 4 weak acid acid pH 6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 alkali Demonstrate your Learning for Outcome 1 Apply (L5) Can you use a storey board to explain how to identify if a household substance is an acid, alkali or neutral Create I am working at level .... because ........... Evaluate Understand (L4) Analyse Apply Understand Remember 07:38 Did you: 1. Complete the experiment ? Can you classify different household substances into acid or alkali? Extended Learning Extended Learning task: • Make a poster display of all the different acids and alkalis in the home and their different uses. • e.g. Vinegar is an acid and it can be used in homes to make pickles. Due date: Criteria for Level 5: – Basic description, basic detail. Criteria for Level 6: – Description with explanation and good level of detail. Criteria for Level 7: – Detailed description and in depth detailed explanation using examples to highlight points made. 07:38 Learning Outcome 1: Review To carry out an experiment to identify and classify household substances into acids and alkalis Go back to your Learning Outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Learning Outcome Learning Outcome 1: Level 5 07:38 How I did Met? Partly met? Not met? Targets How can I improve on Learning Outcome 1? New Information for Learning Outcome 2 What are hazard symbols? How can you tell which chemicals are safe and which are dangerous? Special symbols are used on bottles and vehicles that contain dangerous chemicals. i toxic irritant h harmful corrosive highly flammable These hazard symbols show why the chemical is dangerous. Why is it important that these symbols can be recognised by people from other countries? 7E Acids and alkalis - Concentrated and dilute Compare the particle model of a concentrated acid with a weak acid. concentrated acid weak acid Which sentence best describes which acid? A. This acid has only a few acid particles compared to water particles. B. This acid has a lot of acid particles compared to water particles. Why is a dilute acid less hazardous than a concentrated acid? Demonstrate your Learning for Outcome 2 I am working at level .... because ........... Create Evaluate To get to the next level I need to ........... Analyse (L6) Apply (L5) Analyse Did you: Apply 1. Do a storey board to explain what happen when UI is added to a substance Understand 2. Identify the colour change with UI? Remember 07:38 Did you: 1. Draw a poster to identify hazard warning symbols 2. Include pictures of the chemicals and information about how to use it safely in the lab? 3. Explain why dilute acid is less hazardous Learning Outcome 2: Review To carry out an experiment to investigate the heating effect of different bunsen flames. Go back to your Learning Outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Learning Outcome Learning Outcome 2: Level 6 07:38 How I did Met? Partly met? Not met? Targets How can I improve on Learning Outcome 2? Learning Activities for Outcome 3 Extension – Linking the strength of an acid to how corrosive it is Task 7: Draw a line, and add substances to it to show how hazardous or corrosive you think the household substances are Task 2: Extension Name a very harmful acid and very harmful alkali Task 3: Answers h Harmful and corrosive Strong acids and Alkalis Not harmful Weak acids and alkali Learning Outcome 3: Review Go back to your Learning Outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Learning Outcomes Learning Outcome 3: Level 7 07:38 How I did Met? Partly met? Not met? Targets How can I improve on Learning Outcome 3? Review for Remembering • Stand up if you have met the lesson outcomes? • If not what do you need to do next in order to meet the outcome? Record this in your diary as part of your homework. • Is there any part of the lesson you think you need to go over again next lesson? • Tell the person next to you three things you have learnt this lesson. • How will you remember this for your exam? 07:38 Technician’s list Apparatus and chemicals Eye protection Universal Indicator • fizzy drinks • tap water • de-ionised/distilled water • toothpaste • shampoo • soap • vinegar • lemon juice Technical notes 1 Provide small amounts of the samples on watch glasses. The watch glasses can be placed on a white tile marked with the name of the substance - use a waterproof marker. 2 The samples can be arranged around the room so that the students visit each in turn. If any solid sample is used, moisten it with a little de-ionised water.