C3 – Chemicals in Our Lives Revision
advertisement

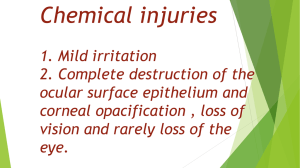
C3 – Chemicals in Our Lives Starter • Element or Compound? Sulphur S Carbon Dioxide CO2 Water H2O Chlorine Cl2 Sulphuric Acid H2SO4 Carbon (Buckminsterfullerene) C60 Sodium Hydroxide NaOH Definitions • Element • Contains only one type of Atom. • Found on the Periodic Table (of elements) • Compound • Consists of 2 or more elements bonded (fixed) together Which Elements are in the Compounds? Carbon Dioxide CO2 Water H2O Sulphuric Acid H2SO4 Sodium Hydroxide NaOH Elements, Compounds and Rocks • Rocks are a MIXTURE of Compounds • The most common compound is Silicon Dioxide SiO2 • This is commonly known as Quartz or Silica Rocks and Plate Movements The Rock Cycle Three Rock Types: Sedimentary - made from sediment Metamorphic – changed by heat and pressure Igneous – formed from Lava or Magma (molten rock) Plate Movements Useful Resources from Rocks • Coal (carbon) • Limestone (Calcium Carbonate) • Marble (Calcium Carbonate) • Rock Salt (Sodium Chloride) • Metal Ores (Compounds containing Metals) Early Chemical Industry What’s the use in Salt? • What can Salt be used for? • List as many things as you can. • Hint – For some, Think about the elements that Salt is made from. Uses of Salt • Food (seasoning and preservation) • Gritting Road (melts ice) • Making Chlorine – Bleach • Making Sodium Hydroxide – Soaps and Cleaners • Making Hydrogen Gas – A Fuel How is Salt obtained? • Evaporation of Sea Water • Mining of Rock salt • Solution mining of Rock Salt Why use salt in our food? • Preservative (stops bacteria from growing) • Flavour enhancer (seasoning) • Makes food taste better / stronger Salted Cod – Used to Preserve it What is Risk? • Risk depends upon 2 factors: • Chance of something happening • Level of harm that occurs if it does Risk • Actual Risk – A Risk calculated from actual data • Perceived Risk – A risk thought by individuals without clear data to support it Precautionary Principle • If the risks or harm from an activity may be greater than any benefit, it makes sense to restrict or stop the activity. • This is particularly true where the level of risk is as yet unknown. Salt and Health • Risks: High Blood Pressure, Heart Disease and Strokes • High Salt diet – Processed food with added salt • Salt is needed – but no more than about 4g per day • Current average intake in the UK: • Men - 11g of salt per day • Women - 8g of salt per day What is an Alkali? • Chemical Compounds that contain HYDROXIDE ions (OH-) • Turn Universal Indicator Blue or Purple • Have a pH over 7, usually between 9 (weak) and 14 (very strong) Recognising Alkalis Uses of Alkalis • Making Soap • Neutralising Soil • Making Glass • Dyeing Cloth Alkali Reactions Is it an Alkali? Sodium Hydroxide Is it an Alkali? Water Is it an Alkali? It turns Universal Indicator Blue Is it an Alkali? Potassium Hydroxide Is it an Alkali? Hydrochloric Acid Is it an Alkali? Urea (Urine) Is it an Alkali? Wasp Sting Is it an Alkali? Soapy Water Is it an Alkali? Milk Is it an Alkali? Ammonium Hydroxide Is it an Alkali? Rain water Is it an Alkali? It turns Universal Indicator Yellow Is it an Alkali? Lithium Hydroxide Is it an Alkali? Sulphuric Acid Is it an Alkali? Blood Is it an Alkali? Bee Sting Is it an Alkali? Bleach Is it an Alkali? Fizzy Drinks Is it an Alkali? Toothpaste Acid or Alkali? Magnesium Hydroxide Acid or Alkali? It turns Universal Indicator Blue Is it an Alkali? Lemon Juice Acid or Alkali? Vinegar Acid or Alkali? Drain Cleaner Acid or Alkali? Bar of Soap Acid or Alkali? Stomach Juices Acid or Alkali? Aluminium Hydroxide Acid or Alkali? It turns Universal Indicator Yellow Acid or Alkali? Caesium Hydroxide Acid or Alkali? HCl Acid or Alkali? NaOH Acid or Alkali? KOH Acid or Alkali? H2SO4 Electrolysis • Using Electricity to split up compounds into the original element that they are made from Chlorine • Extracted from Salt Water by ELECTROLYSIS • Toxic Green Coloured Gas • Very Strong Bleach • Why would it be added to drinking water? Risks of Chlorine in Drinking Water • Chlorine in drinking water can pose potential risks – creating chemicals which are toxic or even carcinogenic • SO WHY ADD CHLORINE??? • Benefit outweighs the risk Questions • Why is it important that drinking water is treated with Chlorine? • Chlorine is a Toxic gas. How can it be safe to add it to drinking water? • What potential health risks come from Chlorine in drinking water? • Why do we continue to add Chlorine, despite the risks is poses? Electrolysis of Brine • Brine – Solution of Salt Water • Salt – Sodium Chloride • Electrolysis – Splitting up compounds using Electrical Current Chemical Warning Signs • Chemistry and Chemicals have their own warning signs of potential hazards • These help you to take the correct precautions to ensure that you stay safe and risk is minimised. • What do these hazard symbols stand for? Flammable Toxic Explosive Corrosive Harmful (h) or Irritant (i) Oxidising Dangerous to the Environment Assessing Chemical Risk • We need to know: - How much of it is needed to cause harm - How much will be used - How it will be used - Chance of escaping into the environment - Who or what it may affect Chemical Safety REACH Regulation Evaluation Authorisation and restriction of Chemical Substances Risks of Plasticisers • uPVC – Stiff, Tough Plastic used to make many things including Drainpipes, Doors and Window Frames • PVC – Can be softened by adding PLASTICISERS. These make the plastic softer and more flexible. Used in sheeting, coating for wires and as a leather replacement. Risks of Plasticisers What does Sustainable mean? • Using the Earth’s resources in a way that can continue in the future • “They won’t run out” At the end of the Life Cycle Life Cycle Assessment CRADLE: What is your product? What materials is it made from? What raw materials are used? Are they sustainable? LIFE: What is your product used for? How long will it last? Is any energy or chemicals used in maintaining it during its life? How long is its life likely to be? GRAVE: What could happen to it at the end of its life? List all the alternatives and what would happen to it in this stage. Which of these is the best option and why? LCA Revision Summary Revision Summary Revision Summary Revision Summary Revision Summary