Density Lab _4 - Moanalua Middle School
advertisement

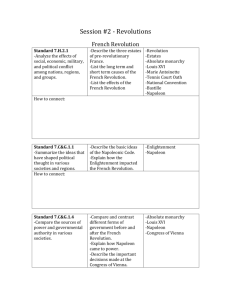
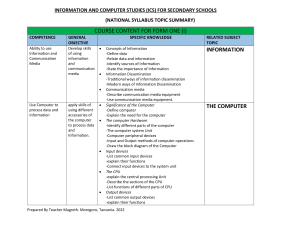
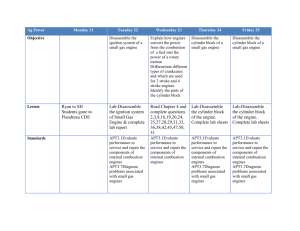
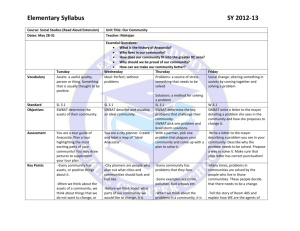
Density Lab #4 April 2 – April 9 Lab Report must be in the correct format •Title and headings •Sections should be in the correct format and complete Title: Density Lab #4 Research Question: How can the density help us identify the material something is made of? Background information: Density: The amount of matter in a given amount of space (characteristic property) Mass: The amount of matter in an object Volume: The amount of space in an object Density = Mass Volume Equations/Formulas: • Density = M or g = balance V ml graduated cylinder • Volume of a cylinder = pi(r2) x h • Volume of a rectangular prism = l x w x h • Volume of a triangular prism = ½ (l x w x h) Hypothesis: • If ………then….. (use what you know from research question and background info) • Object #1 • Object #2 • Object #3 • Object #4 • Object #5 Materials • • • • • • • • 5 objects Centimeter Ruler Water Triple Beam Balance Overflow Can Graduated Cylinder Napkins Calculator Procedure Part 1 1. 2. 3. 4. 5. 6. 7. 8. 9. Find the mass using the balance Find volume using water displacement Find volume using ruler measurements and calculate the volume using the appropriate formula Calculate density m/v using the water displacement value Calculate density m/v using the calculated volume Record data in chart Determine what type of material each sample is made of, based on the known densities of materials Wash hands, clean up Type group data on the computer for the class data set Proper BALANCE use –Calibrate balance to zero –Never place wet objects on balance –Rest the swing when not in use –Move larger weights first –Make sure that the weights are set in the groove –One person moving weights at a time –Let the balance stop swinging on its own Data Observations Object Trial 1 2 1 2 1 2 1 2 1 2 1 2 Mass (g) Volume (H20) displacement Volume (cm3) Density m/v (H20) displacement Density m/v (Calculated Volume) Data Analysis: • Analyzing class data chart – – Determine which pieces of data could possibly be outliers – Find the average density for all remaining data, for each object Compare your averages to the known densities to figure out what your metal cube is possibly made out of H2O 1.00g/ml Magnesium 1.74g/ml Berilium 1.84 g/ml Aluminum 2.70g/ml Titanium 4.5g/ml Zinc 7.13g/ml Tin 7.31g/ml Iron 7.87g/ml Copper 8.96g/ml Silver 10.49g/ml Lead 11.36g/ml Mercury 13.55g/ml Gold 19.32g/ml Platinum 21.46g/ml Brass 7.3 - 8.4 g/ml Steel 7.7 - 8.1g/ml Sterling Silver 10.2-10.3g/ml Compare your averages to the known densities to figure out what your metal cube is possibly made out of H2O 1.00g/ml Magnesium 1.74g/ml Berilium 1.84 g/ml Aluminum 2.70g/ml Titanium 4.5g/ml Zinc 7.13g/ml Tin 7.31g/ml Iron 7.87g/ml Copper 8.96g/ml Silver 10.49g/ml Lead 11.36g/ml Mercury 13.55g/ml Gold 19.32g/ml Platinum 21.46g/ml Brass 7.3 - 8.4 g/ml Steel 7.7 - 8.1g/ml Sterling Silver 10.2-10.3g/ml Conclusion: -Restate Problem -Define a Characteristic Property -Answer the research question -Explain a real world example of density -Restate the hypothesis -Validate or Refute the hypothesis -Explain how the evidence validates or refulte the hypothesis -Explain how the evidence answers the problem -Error Analysis -Improve Lab