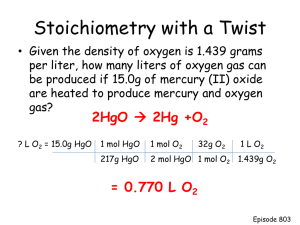Performing a Mass-Mass Stoichiometry Calculation
advertisement

Performing a Mass-Mass Stoichiometry Calculation A step-by-step tutorial Question Statement: What mass of iron(III)oxide will be produced when 15.0 grams of iron are reacted with excess oxygen gas? iron + oxygen iron (III) oxide • Step 1 Stoichiometry problems always begin with a balanced equation. In this problem, we will need to first write formulas for the substances above and then determine the coefficients that balance the equation. What mass of iron(III)oxide will be produced when 15.0 grams of iron are reacted with excess oxygen gas? 4 Fe + 3 O2 2 Fe2O3 The above equation now shows the correct formulas for the reactants and products and is also balanced with the correct coefficients. The coefficients represent a mole ratio of 4 : 3 : 2 in this reaction. What mass of iron(III)oxide will be produced when 15.0 grams of iron are reacted with excess oxygen gas? 4 Fe + 3 O2 2 Fe2O3 15.0 g ? g • Step 2 Next, we will convert the starting mass of 15.0 g of Fe into moles. Here is what we write: 15.0 g 1m ol F e Fe 55.845 g F e What mass of iron(III)oxide will be produced when 15.0 grams of iron are reacted with excess oxygen gas? 4 Fe + 3 O2 2 Fe2O3 15.0 g ? g • Step 3 Now, we are ready to use the mole ratio. The mole ratio converts the substance of known mass into the substance of unknown mass. Here is what we write: 15.0 g 1m ol F e Fe 55.845 g F e 2 m ol F e2 O 3 4 m ol F e What mass of iron(III)oxide will be produced when 15.0 grams of iron are reacted with excess oxygen gas? 4 Fe + 3 O2 2 Fe2O3 15.0 g ? g 15.0 g 1m ol F e Fe 55.845 g F e 2 m ol F e2 O 3 4 m ol F e Notice how the ratio in the balanced equation is transferred into the calculation. What mass of iron(III)oxide will be produced when 15.0 grams of iron are reacted with excess oxygen gas? 4 Fe + 3 O2 2 Fe2O3 15.0 g 15.0 g ? g Fe 1 m ol 55.845 Fe g Fe 2 m ol F e2 O 3 4 m ol F e The ratio is written with the moles of Fe in the denominator so that the unit label of “moles Fe” will cancel out. What mass of iron(III)oxide will be produced when 15.0 grams of iron are reacted with excess oxygen gas? 4 Fe + 3 O2 2 Fe2O3 15.0 g ? g • Step 4 Finally, we convert the moles of iron(III)oxide into grams using its known molar mass. Here is what we write: 15.0 g 1m ol F e Fe 55.845 g F e 2 m ol F e2 O 3 159.866 g F e2 O 3 4 m ol F e 1m ol F e2 O 3 What mass of iron(III)oxide will be produced when 15.0 grams of iron are reacted with excess oxygen gas? 4 Fe + 3 O2 2 Fe2O3 15.0 g 15.0 g Fe ? g 1 m ol 55.845 Fe g Fe 2 m ol F e2 O3 4 m ol F e 159.866 g F e O 2 3 1 m ol F e2 O3 Once again, the units are placed into the calculation so that they will cancel out and leave us with the units we want in the end. What mass of iron(III)oxide will be produced when 15.0 grams of iron are reacted with excess oxygen gas? 4 Fe + 3 O2 2 Fe2O3 15.0 g 15.0 g Fe 1 m ol 55.845 ? g Fe g Fe 2 m ol F e2 O3 4 m ol F e 159.866 g F e O 2 3 1 m ol F e2 O3 21.4 g F e2 O3 Performing the calculation results in the mass of iron(III)oxide that answers the question. What mass of iron(III)oxide will be produced when 15.0 grams of iron are reacted with excess oxygen gas?