2013 Consolidated ARV Guidelines
OVERVIEW OF CLINICAL RECOMMENDATIONS FOR ADULTS,
PREGNANT WOMEN AND CHILDREN
Dr. Philippa Easterbrook
Dr. Philippa Easterbrook
Excellent healthcare – locally delivered
30th June 2013
Guideline Dissemination
Regional Guidelines Workshops
Month
Event
Dates
Location
July
Strategic Use of ARVs
23-25/07/2013
Yogyakarta, Indonesia
July
AFRO East and Southern
Africa
23-25/07/2013
Pretoria, South Africa
August
PPTCT Asia Regional
PMTCT meeting
27-29/08/2013
Kathmandu, Nepal
August
PAHO Regional
26-28/08/2013
Buenos Aires, Argentina
September
EMRO Regional
11-13/09/2013
Casablanca, Morocco
September
WPRO/SEARO Consultation
16-18/09/2013
Beijing, China
EURO
29-31/10/2013
Istanbul, Turkey
November
AFRO East and Central
Africa
11-13/11/2013
Accra, Ghana
December
ICASA
7-11/12/2013
Cape Town, South
Africa
October
Objectives of presentation
• WHO guidelines development and key features
• Recommendations for Adults, pregnant women and
children and Overview of Evidence Base and
Rationale:
• When to Start ART
• What ART to Start (First-Line)
• What ART to Switch to (Second-Line)
• How to Monitor ART
Find the New 2013 WHO Consolidated ARV Guidelines on
http://www.who.int/hiv/pub/guidelines/arv2013/en/index.html
30th June 2013
Evolution of WHO ART Guidelines
TOPIC
WHEN TO
START
2002
CD4 ≤200
2003
2006
CD4 ≤ 200
Since 2001
4 weeks AZT; AZT+
3TC, or single
dose NVP
2004
AZT from 28
weeks
+ single dose
NVP
2010
CD4 ≤ 200
- Consider 350
- CD4 ≤ 350 for TB
CD4 ≤ 350
-Irrespective CD4 for TB &
HBV
AZT from 28 weeks
+ single dose NVP
+AZT/3TC 7days
Option A
(AZT +infant NVP)
Option B
(triple ARVs)
Earlier initiation
PMTCT
Vitoria M et al, Curr Opin HIV/AIDS 2013
Simplified treatment options for pregnant women
1ST LINE
8 options
- AZT
preferred
4 options
- AZT preferred
8 options
- AZT or TDF
preferred
- d4T dose reduction
6 options & FDCs
- AZT or TDF preferred
- d4T phase out
Boosted PI
ATV/r, DRV/r, FPV/r
LPV/r, SQV/r
Boosted PI
Heat stable FDC: LPV/r,
ATV/r
Yes
Tertiary centers
Yes
Phase in
Simpler treatment
2ND LINE
Boosted PI
Boosted PI
Less toxic, more robust regimens
VIRAL
LOAD
TESTING
No
No
(Desirable)
Better monitoring
WHO 2013 Consolidated ARV Guidelines
WHAT TO DO?
• When to start or
switch
• Which regimen to
use
• How to monitor
• Co-infections &
co-morbidities
Clinical
Operational
Guidance for
Programme
Managers
HOW TO DECIDE?
• Prioritization
• Equity and ethics
• Monitoring & Evaluation
HOW TO DO IT?
• Service delivery
• Diagnostics
• Drug supply
Concept Behind Consolidation…
•
•
•
Consolidation across populations and ages
Consolidation along the continuum of care
Consolidation of new with existing guidance
WHO Guideline Development
1
Scoping the document
2
3
Setting up Guideline Development Group and
External Review Group
Disclosure and management of secondary interests
4
Formulation of the questions (PICO) and
choice of the relevant outcomes
5
Evidence retrieval, assessment and synthesis
(systematic review(s))
GRADE - evidence profile(s)
6
Formulation of the recommendations (GRADE)
Including explicit consideration of:
Benefits and harms
Values and preferences
Resource use
7
Dissemination, implementation
(adaptation)
8
Evaluation of impact
9
Plan for updating
PICO: requires specifics of
Population , Intervention,
Comparator & Outcomes
Guideline Development at WHO
1
Scoping the document
2
3
Setting up Guideline Development Group and
External Review Group
Disclosure and management of secondary interests
4
Formulation of the questions (PICO) and
choice of the relevant outcomes
5
Evidence retrieval, assessment and synthesis
(systematic review(s))
PICO: requires specifics of
Population , Intervention,
Comparator & Outcomes
GRADE - evidence profile(s)
6
Formulation of the recommendations (GRADE)
Quality assessment
Including explicit consideration of:
Benefits and harms
Values and preferences
Resource use
7
8
9
Plan for updating
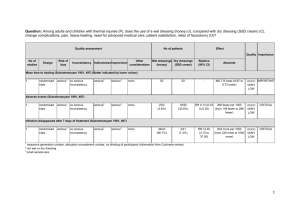
Effect
350+
<350
127/886
(14.3%)
119/877
(13.6%)
RR 1.06
(0.84 to
1.33)
Grade 3 or 4 lab abnormalities
1
randomized no serious no serious
trials
risk of bias inconsistency
none
242/886
(27.3%)
161/877
(18.4%)
RR 1.49
(1.25 to
1.77)
no serious
indirectness
no serious
imprecision
Relative
(95% CI)
Quality Importance
Absolute
8 more per 1000
(from 22 fewer to 45 HIGH
more)
CRITICAL
HIGH
CRITICAL
90 more per 1000
(from 46 more to
141 more)
Serious non-AIDS events and non-opportunistic diseases
1
randomized no serious no serious
serious
4
trials
risk of bias inconsistency
indirectness
very serious
3
none
2/249
2
(0.8%)
12/228
2
(5.3%)
RR 0.14
(0.03 to
0.64)
45 fewer per 1000
(from 19 fewer to 51 VERY
fewer)
LOW
CRITICAL
Progression to AIDS
1
randomized no serious no serious
trials
risk of bias inconsistency
serious
4
indirectness
very serious
3
none
4/364
2
(1.1%)
11/314
2
(3.5%)
RR 0.31
(0.10 to
0.96)
24 fewer per 1000
(from 1 fewer to 32
fewer)
VERY
LOW
CRITICAL
Death or progression to AIDS
2
randomized no serious no serious
trials
risk of bias inconsistency
serious
4
indirectness
serious
none
44/1257
2
(3.5%)
76/1196
2
(6.4%)
RR 0.48
(0.26 to
0.91)
33 fewer per 1000
(from 6 fewer to 47
fewer)
LOW
CRITICAL
Viral Failure (ABC/3TC)
1
randomized no serious no serious
trials
risk of bias inconsistency
no serious
indirectness
very serious
3
none
25/204
2
(12.3%)
25/324
2
(7.7%)
RR 1.54
(0.92 to
2.56)
42 more per 1000
(from 6 fewer to 120 LOW
more)
CRITICAL
Viral Failure (TDF/FTC)
1
randomized no serious no serious
trials
risk of bias inconsistency
no serious
indirectness
very serious
3
none
20/204
2
(9.8%)
25/324
2
(7.7%)
RR 1.30
(0.77 to
2.20)
23 more per 1000
(from 18 fewer to 93 LOW
more)
CRITICAL
no serious
indirectness
very serious
3
none
10/893
2
(1.1%)
13/882
2
(1.5%)
RR 0.77
(0.34 to
1.75)
3 fewer per 1000
(from 10 fewer to 11 LOW
more)
CRITICAL
Dissemination, implementation
(adaptation)
Evaluation of impact
No of patients
No of
Risk of
Other
Design
Inconsistency
Indirectness
Imprecision
studies
bias
considerations
SAEs
1
randomized no serious no serious
no serious
no serious
none
trials
risk of bias inconsistency
indirectness
imprecision
Death
1
randomised no serious no serious
trials
risk of bias inconsistency
1
Grades of Recommendation Assessment, Development and Evaluation
By outcome:
QUALITY OF THE
EVIDENCE
•
•
•
•
High quality
Moderate
Low
Very low
Strong or Conditional depends on:
STRENGTH OF
RECOMMENDATION
•
•
•
•
•
Quality of evidence
Balance of benefits and harms
Values and preferences
Resource use
Feasibility
Guideline development at WHO
1
Scoping the document
2
3
Setting up Guideline Development Group and
External Review Group
Disclosure and management of secondary interests
4
Formulation of the questions (PICO) and
choice of the relevant outcomes
5
Evidence retrieval, assessment and synthesis
(systematic review(s))
GRADE - evidence profile(s)
6
Formulation of the recommendations (GRADE)
Including explicit consideration of:
Benefits and harms
Values and preferences
Resource use
7
Dissemination, implementation
(adaptation)
8
Evaluation of impact
9
Plan for updating
PICO: requires specifics of
Population , Intervention,
Comparator & Outcomes
Modelling of impact and cost-effectiveness
•
•
•
Earlier ART and different testing
strategies
Different populations
General population
Serodiscordant couples
Pregnant women
IDUs, sex workers and MSMs
HIV-HBV and HCV
Different settings
Generalised (South Africa, Zambia)
Concentrated (Vietnam, India)
Values and Preferences
Community consultation
•
•
•
•
E-survey (n=1088), E-forums
(n=955)
6 UN languages
E-survey: 21% LIC, 58% MIC; 45%
PLHIV;
Topics
Earlier ARV initiation
Lifelong ART in pregnant women
Task-shifting and integrated
services
Role of communities
Option B+ Focus Groups
• 87 participants
• Malawi and Uganda
Health care worker consultation
Adult (n=98) & Paediatric
(n=342):
•
9 Global implementers
(ANEPA, ANECA, CHAI, CDC,
EGPAF, ICAP, IeDEA, MSF,
PATH)