SRS Protocol Review Flow Chart
advertisement
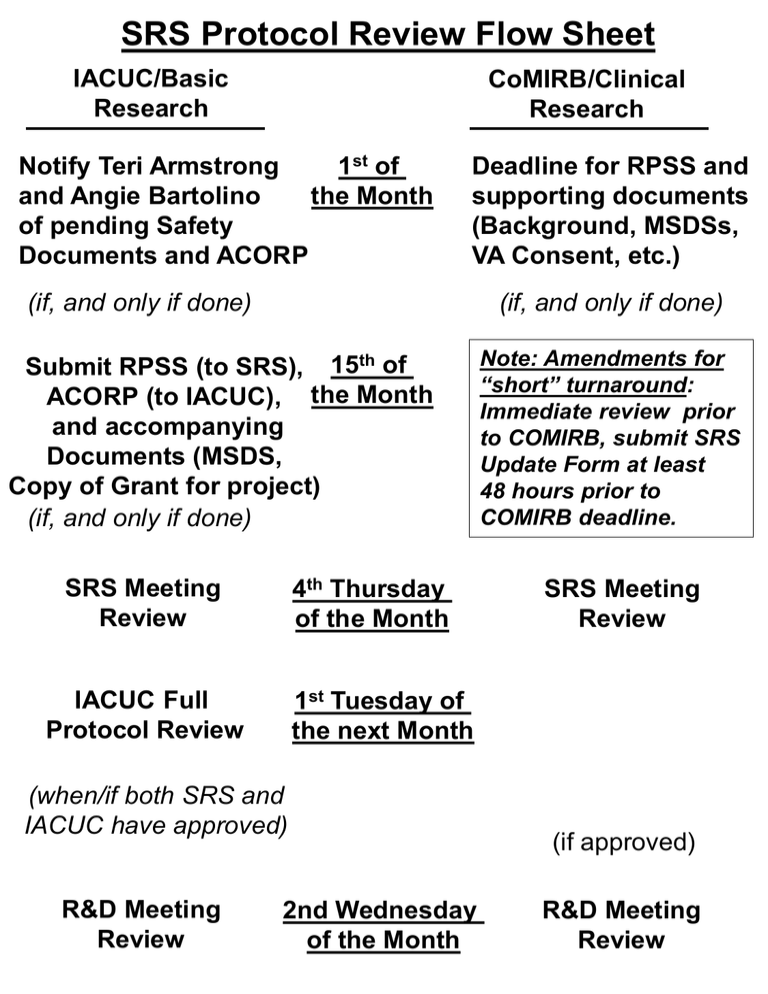
SRS Protocol Review Flow Sheet IACUC/Basic Research CoMIRB/Clinical Research Notify Teri Armstrong 1st of and Angie Bartolino the Month of pending Safety Documents and ACORP Deadline for RPSS and supporting documents (Background, MSDSs, VA Consent, etc.) (if, and only if done) (if, and only if done) Submit RPSS (to SRS), 15th of ACORP (to IACUC), the Month and accompanying Documents (MSDS, Copy of Grant for project) (if, and only if done) 4th Thursday of the Month SRS Meeting Review SRS Meeting Review 1st Tuesday of the next Month IACUC Full Protocol Review (when/if both SRS and IACUC have approved) R&D Meeting Review Note: Amendments for “short” turnaround: Immediate review prior to COMIRB, submit SRS Update Form at least 48 hours prior to COMIRB deadline. 2nd Wednesday of the Month (if approved) R&D Meeting Review Important information to remember: 1. ALL SRS documents should be submitted at the same time, in the same email. We handle approximately 30 applications per month, with an average of 4 documents per submission. Tracking all these separately, particularly as this is NOT in our primary job description, is tedious and time consuming. Only Word copies of RPSS documents will be accepted. 2. Regarding the new Expedited IACUC Review procedure, all IACUC ACORP documents, including SRS RPSS and other necessary safety documents, must be submitted to Teri Armstrong in a single email by the 15th of the month. If the entire packet is not present, the protocol will not be reviewed. 2. All deadlines on the flowchart are immutable; if you miss the deadline your protocol will not be considered until the next month’s review cycle. There are no emergencies, just poor planning. 3. Amended protocols (IACUC or CoMIRB) that are not consistent with the currently approved RPSS (or that do not have an associated RPSS) need to have a revised or new RPSS that is consistent with the proposed work. Example: if your IACUC protocol/RPSS do not specify blood and animal tissue removal, but you are now amending the protocol to take blood samples, you will have to revise the RPSS to indicate new risks and appropriate methods to alleviate the risk. Don Lawrence can help you with such revisions.