IACUC Seminar Presentation by Dan Rule, Ph.D.
advertisement

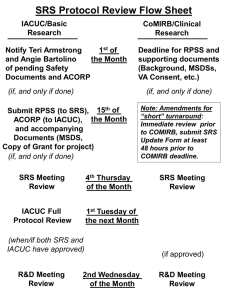
University of Wyoming Institutional Animal Care and Use Committee Scientist Involvement 1950- Animal Care Panel (ACP) 1951- Proceedings of the ACP published 1952- Institute of Laboratory Animal Resources 1960- Animal Facilities Certification Board 1963- Guide for Laboratory Animal Facilities and Care 1965- AAALAC incorporated 1965- 2nd edition of “The Guide” published; 7th edition published in 1996 1967- ACP became AALAS Government Involvement 1962- NIH Contract – First “Guide” 1966- Laboratory Animal Welfare Act 1970- Animal Welfare Act (’70, ’76, ’85’ 90, 2002) 1971- NIH “Policy” 1973- Public Health Service Policy (’79, ’85) 1985- PHS Act (Health Research Extension Act) 1966 Laboratory Animal Welfare Act Focus on preventing theft of dogs and cats Holding areas emphasized Only developed specific areas of standards Housing, Feeding, Watering, Sanitation, Shelter, Separation of species, Ventilation, Veterinary care Animal Welfare Act 1970 the LAWA changed to AWA Extended to the laboratory Reported number of animals by pain category Required appropriate use of anesthetics No ACUC yet 1971 NIH Policy Institutions had to have “Assurances” Annual inspections Compliance with AWA Follow the “Guide” 1973 Public Health Service Policy followed 1979 PHS highlights IACUC now required Assurance required before funding received All live vertebrates Annual reports required Recommended to have protocol reviews 1985 AWA Amendments Established the IACUC Assigned responsibility to Institutional Official (IO) Protocol review Semiannual inspection and program review Search for alternatives to painful procedures Personnel qualifications Environmental enrichment Exercise for dogs 1985 PHS Policy More detailed Assurance IO ultimately responsible IACUC members are appointed by CEO Minimum of 5 members Protocol reviews required Semiannual reports of program review Detailed training program description UW IACUC Bill Gern, Institutional Official Dorothy Yates , Liaison to Institutional Official Linda Osterman, Coordinator Colette Kuhfuss, Coordinator Dave Evertson, Attending Veterinarian Dan Rule, IACUC Chair, Animal Science Scott Seville, Zoology and Physiology Sreejayan Nair, School of Pharmacy Myrna Miller, Veterinary Sciences Bob Boysen, Unaffiliated member Ashley Guritza, UW General Council, Tara Evans, UW General Council IACUC Protocol Preparation FOR IACUC USE Approved for period (one year maximum) Approved ______________ to ________________ Copy to PI ________________________________ Copy to Animal Care Worker _________________ DHHS/NIH/OLAW ASSURANCE #A-3216-01 EFFECTIVE: 08/26/05-08/31/09 UNIVERSITY of WYOMING INSTITUTIONAL ANIMAL CARE and USE COMMITTEE PROTOCOL APPROVAL FORM Submit completed form to the IACUC, Office of Research, Room 308, Old Main. RESEARCH SHALL NOT BEGIN BEFORE THIS FORM IS APPROVED. New project ____________ Revised protocol ____________ Date________ Title of project : Project leader(s): Department(s): Phone: Email: Type: Research _____ Instruction ______ Location of animal room: Building/Room number Location of animal care log book/medical records: Building/Room number Duration of project: Begin End (Project will be approved for one year from date of IACUC approval. An update form must be submitted annually if project extends beyond one year.) Sponsoring agency (if applicable): UW Budget ID/Project Grant number (if applicable): Name person(s) and/or unit responsible for animal care: Name: Phone: Animal species (genus, species, common name) (one species per protocol form): Number to be used/year: Total animal days/year (# animals x #days): Number to be used/project: Total animal days/project: Source of animals: Commonly overlooked Animal species (genus, species, common name) (one species per protocol form): Number to be used/year: Total animal days/year (# animals x #days): Number to be used/project: Total animal days/project: Source of animals: Is simple math, but quite often the number of animals indicated on this page does not match up with the numbers indicated in the description of the research Proposal category: A _____ Live animals will be humanely killed without any treatments, manipulations, etc., but will be used to obtain tissue, cells, sera, etc. B _____Live animals will have manipulations, surgery, etc. performed while anesthetized. The animals will be humanely killed at termination of the experiment without regaining consciousness (non-survival surgery). C _____ Live animals will receive a painful stimulus of short duration without anesthesia behavior experiments with flight or avoidance reactions) resulting in a short-term traumatic response. Other examples in this category are blood sampling, injections of adjuvant, etc. D _____ Live animals will have significant manipulations, surgery, etc., performed while anesthetized and will be allowed to recover (survival surgery). Such procedures (e.g., chronic catheters, surgical wounds, implants) cause a minimum of pain and/or distress. Also included are mild toxic drugs or chemicals, tumor implants (including hybridomas), tethered animals, short-term restrained animals. E _____ Live animals will have significant manipulations, surgery, severe discomfort, etc. without benefit of anesthesia, analgesics or tranquilizers. Examples are toxicity testing, radiation sickness, irritants, burns, trauma, biologic toxins, virulence challenge, prolonged restriction of food or water intake, cold exposure, restraint, or drug addiction. All use of paralytic agents (curare-like drugs) must be included in this category. O _____ Other: Standard husbandry practices, weighing, observation, insemination. Choice of category?? If not clear on which category to check, be conservative Check the next highest category to make sure the project is covering the bases adequately The IACUC review will compare procedures with category chosen 1) a. Purpose (in lay terms) b. Scientific objective(s) Potential for use of in vitro systems or computerized models instead of live animals i. Elaborate on current availability of animal data that could be used to predict outcomes ii. Elaborate on the uniqueness of the study such that the requirement for live animal research is necessary Purpose – in “lay terms” Should be written at a high school level The nature of the study and the relevant benefactors should be indicated For example, if the study is to benefit agricultural production, say so Biomedical Veterinary medical Environmental, zoological, etc. 1) a. Purpose (in lay terms) b. Scientific objective(s) Potential for use of in vitro systems or computerized models instead of live animals i. Elaborate on current availability of animal data that could be used to predict outcomes ii. Elaborate on the uniqueness of the study such that the requirement for live animal research is necessary This is where you justify the use of animals for the research. - “In vitro or comperized models cannot be use because…” - “The current animal data indicates….; however,…..” - “The proposed study is unique because….; therefore, new animal research is necessary to address the problem.” 2) Describe all procedures: Description should allow the IACUC to understand the experimental course of an animal from its entry into the experiment to the endpoint of the study. Overview of procedures (Reference citations for b-e) b. Type and duration of restraint c. Name and dose of anesthesia and/or tranquilizer (contact attending veterinarian) d. Surgical procedures i. pre-operative procedures ii. asceptic methods to be used: surgical attire iii. who will perform surgical procedures iv. where will surgical procedures be performed v. non-survival/survival surgery vi. justification if more than one surgical procedure per animal Post-surgical care i. Recovery facility ii. Name, dose, route of administration and regimen for analgesia; (investigate literature for pain management for species used; consult attending veterinarian) 2) Describe all procedures: Description should allow the IACUC to understand the experimental course of an animal from its entry into the experiment to the endpoint of the study. Overview of procedures (Reference citations for b-e) b. Type and duration of restraint c. Name and dose of anesthesia and/or tranquilizer (contact attending veterinarian) d. Surgical procedures i. pre-operative procedures ii. asceptic methods to be used: surgical attire iii. who will perform surgical procedures iv. where will surgical procedures be performed v. non-survival/survival surgery vi. justification if more than one surgical procedure per animal Post-surgical care i. Recovery facility ii. Name, dose, route of administration and regimen for analgesia; (investigate literature for pain management for species used; consult attending veterinarian) Describe procedures Be thorough Address each item If not conducting surgery, address with “N/A” A “copy and paste” would work here, but be succinct – no need for numerous paragraphs and pages from your grant proposal 3) Justification for species chosen (lowest possible species on phylogenetic scale) “lowest species on the phylogenetic scale” Example – you propose to use sheep Why can’t you use a mouse? Some examples of why have included the specialized GI tract; the presence of a large enough pituitary to study; characteristic reproductive cycles Start the sentence with “The (species) is the lowest species on the phylogenetic scale because……” finish the sentence 4) Statistical justification for the specified number of animals (assistance to determine the appropriate number of animals per treatment) a. Justification for number of animals per experiment b. Justification for number of experiments per year (as stated on page 2) c. Literature cited/reviewed for justification of number of animals proposed Statistical Justification The same statistical justification used in a grant proposal should be used here Examples of what NOT to use include: Number chosen based on number of cages or pens Based on available financial resources Based on what you used last time Statistical Justification If you need to add animals because of expected mortality, say so Don’t add animals just because more would be better The idea is to use enough to obtain data worthy of interpretation Too few or more than necessary could be a red flag Statistical Justification If previous studies demonstrate how the proposed number is appropriate to test your hypotheses, cite the work If the number is larger than capacity, propose to replicate the treatments to achieve the appropriate number If no data are available, propose the study as a pilot trial to obtain base line data that can be used to calculate the appropriate “n” Power tests Power tests are often used to establish “n” Some data are required Pilot studies, literature Statistics books, stat analysis software Research Office web page links Consult (collaborate) with a statistician Power test may not be best approach Will animals be subjected to euthanasia? a. Method of euthanasia b. Drug and dosage c. If using drugs for euthanasia, describe disposal of animal remains. d. If animals will not be euthanized, describe plan for future use or other dispersal. Landfill disposal If euthanasia method includes use of drugs for which residues could be consumed by birds of prey, such as eagles, this could be a problem Indicate that landfill personnel will be instructed to bury the remains immediately There is a charge for the service, so a contract could be needed For rodents, incineration at the Pharmacy facility is a good approach If the proposal category checked is D or E, then the experimental procedures may cause more than momentary or slight pain or distress. Address the following: Provide written narrative description, including methods and sources used in search, of how it was determined that alternatives to potentially painful or distressful procedures are not available. Including i: Literature cited; database references must include name of databases searched, the date of the search, period covered, and keywords used. And/Or ii: personal communications Please refer to Animal Welfare Act 9CFR Section 2.31 (d) (1) (ii) Category D and E Requires the most attention Requires a thorough search for and discussion of alternative procedures Search key words should include in the list “alternatives” along with key words pertinent to the research area Alternative procedures that could be less painful and less stressful might not be appropriate for your study; indicate why Your procedure might be a less painful and stressful modification of published procedures – i.e. the alternative. If so state that it is the alternative Addressing the issue of “D” and “E” category Be thorough Actually look for alternatives Who knows, you might find something useful If not, at least you tried, and this is what is most important for the protocol reviewer Explain why this research does not involve unnecessary duplication of previous research or experiments. Describe the literature search method. a. If using a web-based database search, indicate date of search, name of database, keywords used, and number of responses. b. Discuss relevant literature to justify why unnecessary duplication of previous research is not involved. Please refer to Animal Welfare Act 9CFR Section 2.31 (d) (1) (iii) …..unnecessary duplication…. Appears redundant If the research proposed has not been done before, you would or should know this Indicate the search engine and key words Some studies are closely related, but the hypotheses were clearly different so the approach would be different Plan to “split hairs” as the difference might not be obvious to a reviewer who is not as closely associated with the work as you are – keep the language simple and clear Training/experience documentation: Federal regulations require appropriate training and experience for all personnel involved in the care and use of animals. An up-to-date "Verification of Training for Animal Work" form must be on file in the Research Office for each person, including the P.I., involved in the care and use of animals to be used in this protocol. Verification of Training for Animal Work form: http://www.uwyo.edu/Research/forms.htm For PI(s) (name): attached ___ on file ___ date _____________ For animal care worker/lab technician (name): attached ___ on file ___ date _____________ Graduate student(s) (name): attached ___ on file ___ date _____________ Others (name): attached ___ on file ___ date _____________ Please list specific experience and/or qualifications of each animal care worker necessary to perform the specific techniques and procedures described in this protocol (such as surgery) if not included on the "Verification of Training for Animal Work" form attached or on file. Training Get the training certification from the Research Office web page Fill it out and submit it Once on file, reference this item as “on file” Everyone who is involved in animal research is required to take the CITI online training to be compliant with UW assurance Principle Investigator Assurance: "I have received a copy of the NIH Guide for the Care and Use of Laboratory Animals and/or The Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching and will provide for the care, use and treatment of the animals used for the purpose described above accordingly. I will use procedures which will avoid or minimize discomfort, distress and pain to animals used in my research. I have considered alternatives to procedures that may cause more than momentary slight pain or distress to the animals. These studies do not unnecessarily duplicate previous experiments. I HAVE CONSULTED AS NEEDED WITH ATTENDING VETERINARIAN (David Evertson 7457341), OR BACKUP VETERINARIAN ON STAFF AT ALPINE VETERINARY CLINIC,DURING THE PLANNING OF THIS PROJECT AND WILL CONSULT WITH THE VETERINARIAN DURING THE PROJECT. I will inform the attending Veterinarian immediately if any problems occur, including unanticipated pain or distress, injury, morbidity or mortality. I will submit a revised protocol for IACUC approval if any significant deviation from the approved project procedures occurs. I will submit an annual update for IACUC approval for continuation if this project extends beyond one year. I assure the IACUC that all persons involved in the care and use of animals used to conduct this protocol have received the appropriate training and are qualified to perform the procedures described above." Principle Investigator Assurance: "I have received a copy of the NIH Guide for the Care and Use of Laboratory Animals and/or The Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching and will provide for the care, use and treatment of the animals used for the purpose described above accordingly. I will use procedures which will avoid or minimize discomfort, distress and pain to animals used in my research. I have considered alternatives to procedures that may cause more than momentary slight pain or distress to the animals. These studies do not unnecessarily duplicate previous experiments. I HAVE CONSULTED AS NEEDED WITH ATTENDING VETERINARIAN (David Evertson 745-7341), OR BACKUP VETERINARIAN ON STAFF AT ALPINE VETERINARY CLINIC,DURING THE PLANNING OF THIS PROJECT AND WILL CONSULT WITH THE VETERINARIAN DURING THE PROJECT. I will inform the attending Veterinarian immediately if any problems occur, including unanticipated pain or distress, injury, morbidity or mortality. I will submit a revised protocol for IACUC approval if any significant deviation from the approved project procedures occurs. I will submit an annual update for IACUC approval for continuation if this project extends beyond one year. I assure the IACUC that all persons involved in the care and use of animals used to conduct this protocol have received the appropriate training and are qualified to perform the procedures described above." The assurance By signing, the investigator, department head, ACUC chairman, and IO assure that all indicated will be done Is a contract The University of Wyoming also has submitted assurances that the IACUC must act according to established guidelines Annual Updates If there are no changes, indicate “No Changes” Indicate the number of animals proposed for the previous year from the original protocol Indicate the number actually used If the numbers used and proposed are different, that is a “Change” Indicate the number proposed for upcoming year Reminders and helpful hints on commonly overlooked items: New protocols: ○ Genus and species are indicated. ○ For blood draws/injections: location on animal; volume; needle dimensions are given. ○ If anesthesia and/or euthanasia are proposed the dosages are indicated along with injection location, volume, and needle dimensions. ○ For all personnel on the protocol each has appropriate CITI training completed. Reminders and helpful hints on commonly overlooked items: New protocols: ○ For a category “D” or “E” protocol, the literature search for alternatives has been done with the search key words including the term “alternatives” in the list along with the search engines used. ○ For statistical justification, calculations of “n” are included with reference(s); for pilot studies the verification of lack of sufficient data or published data from other labs is included. Reminders and helpful hints on commonly overlooked items: Annual updates: ○ For annual updates in which the work done was approved as a pilot study, the continuation of the project will likely not be a “pilot” study as sufficient data for calculation of animals numbers; statistical justification of “n” will be needed in the annual update for additional animal experiments. ○ For annual updates, any change in the number of animals is indicated with brief justification. Indicate the number proposed in the original protocol; the number used during the year of the update, and the number to be used in the upcoming year. Please remember that all investigators (even DVMs) are required to contact the Attending Veterinarian if any issues regarding the health and well-being of the animals on protocol occur! Additional Protocols Teaching protocol Breeding colony protocol Wildlife in development Electronic versions planned FOR IACUC USE UNIVERSITY OF WYOMING INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE Required informational sheet for instruction involving the use of animals Semester/ year approved for instruction involving the use of animals: __________ The informational sheet must be filled out and submitted by the faculty member instructing the class using the animals before the first day of class during the semester in which the students will be using the animals. This sheet should be used for class projects or assignments designed to provide students with an opportunity to work with animals as part of a class. If the animals will be used in research, research training, experimentation, biological testing, or exhibition purposes, the faculty member or student must submit a full IACUC protocol. The informational sheet should be submitted to the IACUC in care of: Institutional Animal Care and Use Committee 1000 East University Avenue, Dept. 3355 Room 308, Old Main Laramie, WY 82071 Name: Title: Institution: Department: Phone: 307-766-5320 Fax: 307-766-2608 email: IACUC@uwyo.edu Phone: Fax: Email: Address: 2) Title, semester of class. 3) Purpose for the use of the animals (in lay terms). 4) Description of the procedures, including: - Overview of procedures - Number of animals and species - Type and duration of restraint - Name and dose of anesthesia and/or tranquilizer (contact attending veterinarian) - Surgical procedures and post-surgical care - Justification for species chosen (lowest possible species on phylogenetic scale) - If euthanasia will be used, include (1) the method, (2) the drug and dosage, and (3) the disposal of the animal remains. If animals will not be euthanized, describe plan for future use or other dispersal. 5) Describe the training that will be provided to the students to ensure that they are appropriately qualified and trained to conduct the procedures. 6) Attach a copy of the course syllabus and any other relevant information (e.g., lab manual) Required Training CITI training online Do not have to re-take if done previously elsewhere Required by everyone Summary IACUC is important Contact the ACUC with questions Expect protocol revisions The ACUC will try to make the process as straight forward as possible for the researchers