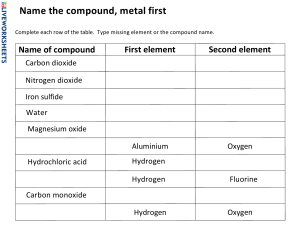
_––– 1. What is the difference between an element and a compound? ______________________________________________________________________ ______________________________________________________________________ ______________________________ 2. Give two examples of elements. ______________________________________________________________________ ______________________________________________________________________ ______________________________ 3. What is a compound? Give an example of one. ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ____________________________________________________________ 4. Is table salt (NaCl) an element, compound, or mixture? Explain why. ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ____________________________________________________________ 5. What is the difference between sea water and pure water? ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ____________________________________________________________ 6. Can a mixture be separated by physical means? Give an example. ______________________________________________________________________ ______________________________________________________________________ ______________________________ 7. What is the chemical formula for water? Is it an element or a compound? ______________________________________________________________________ _______________ 8. How do you classify air: as a mixture, element, or compound? ______________________________________________________________________ _______________ 9. Is sugar (𝐶6 𝐻12 𝑂6) a mixture or a compound? Why? ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ _____________________________________________ 10. How would you classify the human blood?An element, compound or mixture and why? ______________________________________________________________________ ______________________________________________________________________ ______________________________ 11. 12 14.Using the periodic table provided, what is the molecular mass of each of the substances shown below?E.g 𝐻2 0 molecular mass is 18g. i)Hydrochloric acid. HCl _____________________ ii)Ammonia. N𝐻3 iii)Vinegar. C𝐻3 COOH. _____________________ iv)Sulphuric acid. 𝐻2 S𝑂4 _____________________ _____________________ 15.Balance the equations below. ___ Al + O2 ___ Al2O3 ____ Cl2 + ___ KI ____ KCl + ___ I2 ____ Na + ____ O2 ___Na2O ____ K + ____ H2SO4 ____K2SO4 +____ H2 17 Using the diagram below Explain how ionic bonding takes place. ____________________________________________________________________________ ____________________________________________________________________________ ____________________________________________________________________________ ____________________________________________________________________________ ________________________________________________________________ A student conducted the following experiment to show that aluminium reacts with hydroxides to produced hydrogen gas. MATERIALS USED - Aluminum foil (small pieces) - Sodium hydroxide (NaOH) solution (dilute) - Water - 2 conical flasks - Safety goggles and gloves -Lighter or match stick Experiment Part 1: Reaction of Aluminium and Sodium Hydroxide CAUTION: Protect yourself from sodium hydroxide, as it is corrosive. -Mixing: In a conical, add a 20ml of sodium hydroxide solution. -Adding Aluminum: Tear a piece of aluminum foil into small pieces and add it to the sodium hydroxide solution. Observation: You should start to see bubbles forming immediately. This indicates that hydrogen gas is being produced. Experiment Part 2: Water and Aluminium foil In a separate conical flask, just add 20ml of plain water then aluminium foil. Observation:No bubbles or gas should be produced from just water. 16 .Answer the questions below. I) II) III) IV) What are the safety measures that the student must undertake in this experiment? ___________________________________________________________________ ___________________________________________________________________ ________________ What is the purpose of this experiment ? ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ________________________ Suggest a hypothesis for the experiment? ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ________________________________ What is the independent variable in the experiment? ___________________________________________________________________ ___________________________________________________________________ ________________ V) VI) VII) VIII) What is the dependent variable in the experiment conducted ___________________________________________________________________ ___________________________________________________________________ _____________________________ List down at least three controlled variables that MUST be used in the experiment. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ________________________________________________________ How would you test for HYDROGEN gas? ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ __________________________________________ Hydrogen has been used in different countries as an alternative fuel.Explain by applying your knowledge from the experiment,how hydrogen engine works. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ________________________________________________________