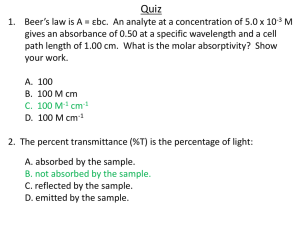
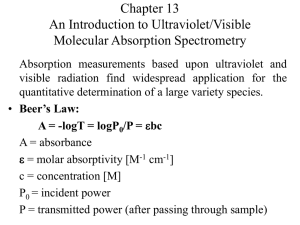
Absorbance and Transmittance • When a beam of radiation of intensity PO is bombarded onto a sample (length b), the intensity of the beam that emerges on the other side of the sample will be PT . • This change in intensity of radiation is attributed to possible absorption by the sample and be described by two quantitative terms, viz, absorbance and transmittance. • Transmittance – amount or ratio of radiation from the source that passes through the sample unabsorbed. 𝑃𝑇 •T= 𝑃𝑂 Absorbance and Transmittance • Absorbance : measure of the amount of light absorbed by a sample. 𝑃𝑇 𝑃𝑂 • A = -log T = - log ( ) = log 𝑃𝑂 𝑃𝑇 • The value of transmittance range from 0 to 1, if there is 100% transmittance, then the value of T = 1. This means 0% absorbance. A = 0. • If there is 0% transmittance, then the value of T is 0. This means 100% absorbance. • If there is 10% transmittance, then the value of T is 0.1. This means 90% absorbance. A = 1 Absorbance and Transmittance • Absorbance can also be related to concentration of the absorbing species (analyte) in a sample and given by the Beer’s law as follows: • A = ƐbC, where Ɛ is molar absorptivity, b is path-length of sample holder and c is molar concentration. • Molar absorptivity units are M-1 cm-1 (litre per mole per cm) pathlength is cm and molar concentration is moles/litre. • A = abc, where a is absorptivity given in litre per gram per cm, path-length is cm and concentration is grams/litre. Calculations examples • Quiz 1: A sample has a %transmittance of 50%. Determine its absorbance. • Answer: 50% transmittance means T has a value of 0.500, thus A = - log T = - log (0.500) = 0.301 • Quiz 2: A 5 × 10-4 M solution of an analyte is placed in a sample cell that has a pathlength of 1.00 cm. When measured at a wavelength of 490 nm, the absorbance of the solution is found to be 0.338. Determine the analyte’s molar absorptivity at this wavelength. Calculations examples • Answer : A = ƐbC 𝐴 Ɛ= 𝑏𝐶 = 0.338 (1.00 𝑐𝑚)(5×10−4 𝑚𝑜𝑙𝑒𝑠 𝑝𝑒𝑟 𝑙𝑖𝑡𝑟𝑒) = 676 M-1 cm-1 • Quiz: Calculate the absorbance and transmittance of a 0.00240 M solution of a substance with a molar absorptivity of 313 M-1 cm-1 in a cell with a 2.00-cm path-length. • Answer: A = ƐbC Calculations examples • A = (313 M-1 cm-1)(2.00 cm)(0.00240 M) = 1.50 • log T = -A • T = 10logT = 10-A = 10-1.50 = 0.0316 • Quiz: A solution prepared by dissolving 25.8 mg of benzene in hexane and diluting to 250.0 mL had an absorption peak at 256 nm and an absorbance of 0.266 in a 1.000-cm cell. Calculate the molar absorptivity of benzene at this wavelength. • Quiz: A sample of hexane contaminated with benzene had an absorbance of 0.070 at 256 nm in a cuvette with a 5.000-cm pathlength. Find the concentration of benzene in mg/L. Multicomponent samples • In a case of multicomponent samples, a calibration curve (abs vs conc) is plotted generated from a series of prepared standard solutions. Standard solutions are prepared in exact manner as the unknown sample. • According to Beer’s law, a calibration curve of absorbance versus the concentration of analyte in a series of standard solutions should be a straight line with an intercept of 0 and a slope of ab or Ɛb. • However in reality most of these calibration curves are non-linear, i.e, they deviate from linearity. Deviations from linearity can be attributed to fundamental, chemical and instrumental limitations. Beer’s law deviation Multicomponent samples • Quiz: The concentration of copper and zinc in the supernatant are determined by atomic absorption using an air-acetylene flame. A series of external standards is prepared and analyzed, the following results are obtained. Standard Concentration (µg/mL) Absorbance 1 2 3 4 5 6 7 8 0.000 0.100 0.200 0.300 0.400 0.500 0.600 0.700 0.000 0.006 0.013 0.020 0.026 0.033 0.039 0.046 Multicomponent samples • After drying and extracting the sample, a 11.23-mg tissue sample gives an absorbance of 0.023. Calculate the concentration (in µg/mL) of copper in the tissue sample using the linear regression method and by extrapolation. Beers law deviation • Instrumental Limitations to Beers Law • There are two principal instrumental limitations to Beer’s law. The first limitation is that Beer’s law is strictly valid for purely monochromatic radiation; that is, for radiation consisting of only one wavelength. • Using polychromatic radiation always gives a negative deviation from Beer’s law. • Stray radiation is the second contribution to instrumental deviations from Beer’s law. Stray radiation arises from imperfections within the wavelength selector that allows extraneous light to “leak” into the instrument and reaches the detector. Beers law deviation • Fundamental Limitations to Beers Law • Beer’s law is a limiting law that is valid only for low concentrations of analyte. • There are two contributions to this fundamental limitation to Beer’s law. • At higher concentrations the individual particles of analyte no longer behave independently of one another. • The resulting interaction between particles of analyte may change the value of Ɛ. Beers law deviation • A second contribution is that the absorptivity, a, and molar absorptivity, Ɛ, depend on the sample’s refractive index. • Since the refractive index varies with the analyte’s concentration, the values of a and e will change. • For sufficiently low concentrations of analyte, the refractive index remains essentially constant, and the calibration curve is linear. Beers law deviation • Chemical Limitations to Beers Law • Chemical deviations from Beer’s law arise when the absorbing species dissociates, associates or reacts with a solvent in an equilibrium reaction to produce a product with a different absorption spectrum than the analyte. • For example in the analysis of a weak acid, HA, the equilibrium shifts to its conjugate weak base A- and both absorb at different wavelengths. HA + H2O H3O+ + A• This shift in equilibrium affects or results in deviation from Beer’s law