Grade 7 Chemistry Revision Worksheet - Atomic Structure & Purity
advertisement

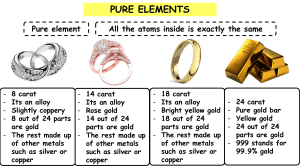
CBSE & Cambridge Assessment International Education REVISION WORKSHEET-1 MARCH 2024 GRADE-7 SUBJECT-CHEMISTRY Name: ___________________________________ Roll no: ______________ Date: ___________ _____________________________________________________________________________________ 1. Read the following statements and identify as ‘true’ or ‘false’. Correct the false statements. a. Electrons have less mass than protons. ………………………………………………………………………………………………………. b. Protons have a negative electrical charge. ………………………………………………………………………………………………………. c. Electrons have a negative electrical charge. ………………………………………………………………………………………………………. d. Neutrons have more mass than electrons. ………………………………………………………………………………………………………. e. Electrons are found in the nucleus of an atom. ………………………………………………………………………………………………………. 2. State one difference between J J Thomson’s model and Rutherford’s model of the structure of an atom. …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… 3. What is meant by ‘Purity’? …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… 4. Calculate the percentage of gold in 14 carat gold chain. 5. 18 carat gold is 75% pure gold and 25% other metals (usually a combination of silver and copper). 9 carat gold, on the other hand, has a content of 37.5% pure gold and 62.5% other metals. CHEMISTRY /REV-WS /MARCH2024 1 It is observed that a ring made of 9 carat gold shows a hardness of 8 on the Mohs scale and a ring made of 18 carat gold shows a hardness of 10. Suggest a possible reason for this observation. …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… 6. Draw a labelled diagram of the Rutherford’s gold foil experiment. 7. Marcus and Zen are investigating how much salt they can dissolve in different volumes of water. Given below are their table of results. Volume of water in cm3 10 Mass of salt in grams 4 20 30 40 50 60 70 9 13 16 20 26 26 a. What is the independent variable in the given investigation? ……………………………………………………………………………………………………. b. Plot a graph and make a line of best fit. CHEMISTRY /REV-WS /MARCH2024 2