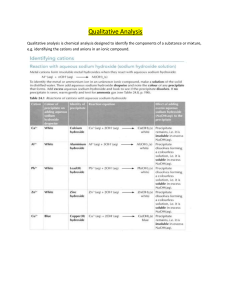
Identification of salts. Salts – ionic substances made of anions (negatively charged ions) and cations (positively charged ions) Testing for cations Cations identified by their reaction with sodium hydroxide solution and aqueous - ammonia. (Both are alkalis and hence produce OH ions which react with the cations being tested). Cations are identified by the formation of precipitates. (These are insoluble solids formed during the reaction of the cation with the alkali). Some cations do not form precipitates with alkalis. The precipitates formed may be soluble or insoluble in excess alkali (i.e when excess alkali is added to the solution containing the cation). The precipitates are formed when a metal ion (cation) reacts with the hydroxide ion – (OH ion) from the alkali. Hence precipitate formed is an insoluble metal hydroxide. + If the cation present is the ammonium cation, NH4 , it produces ammonia gas when heated with the alkali (sodium hydroxide). The ammonia gas can then be tested with moist red litmus paper which turns blue in the presence of ammonia gas. The cation may be identified by the colour of the precipitate formed and also whether the precipitate is soluble or insoluble in excess alkali. When the precipitate is soluble in excess alkali, it forms a complex ion which is soluble. 3+ e.g Al + (aq) - 3 OH (aq) Al(OH)3(s) - OH H + - [Al(OH)4] (aq) complex ion(soluble) Note: All cations form precipitates with sodium hydroxide solution and aqueous ammonia + + + 2+ except Na , K , NH4 . Ca does not form precipitate with aqueous ammonia. 1 Effect of aqueous sodium hydroxide and aqueous ammonia on cations. Cation Reaction with sodium hydroxide solution dropwise and in excess A white ppte of aluminium hydroxide is formed. The ppte is soluble in excess sodium hydroxide solution to give a colourless solution (a complex ion is formed). A white ppte of zinc hydroxide is formed. The ppte is soluble in excess sodium hydroxide solution to give a colourless solution. 3+ Al 2+ Zn A white ppte of calcium hydroxide is formed. The ppte is insoluble in excess sodium hydroxide solution. A dirty green ppte of iron (II) hydroxide is formed, which slowly turns brown when left in air. The ppte is insoluble in excess sodium hydroxide solution. A reddish-brown ppte of iron (III) hydroxide is formed. The ppte is insoluble in excess sodium hydroxide solution. A blue ppte of copper (II) hydroxide is formed. The ppte is insoluble in excess sodium hydroxide solution. 2+ Ca 2+ Fe 3+ Fe 2+ Cu Cr3+ + NH4 + + K & Na A green ppte of chromium (III) hydroxide is formed which is soluble in excess sodium hydroxide to form a green solution. No ppte is formed. On heating, ammonia gas is liberated which turns moist red litmus paper blue. No visible reaction occurs. Reaction with aqueous ammonia dropwise and in excess A white ppte of aluminium hydroxide is formed. The ppte is insoluble in excess aqueous ammonia. A white ppte of zinc hydroxide is formed. The ppte dissolves in excess aqueous ammonia to give a colourless solution (a complex ion is formed). No visible reaction occurs. A dirty green ppte of iron (II) hydroxide is formed. The ppte is insoluble in excess aqueous ammonia. A reddish-brown ppte of iron (III) hydroxide is formed. The ppte is insoluble in excess aqueous ammonia. A blue ppte of copper (II) hydroxide is formed. The ppte dissolves in excess aqueous ammonia to give a dark blue solution (a complex ion is formed) A green ppte of chromium (III) hydroxide is formed which is insoluble in excess aqueous ammonia. No visible reaction occurs. No visible reaction occurs. Identification of anions 2 Anions can be tested in the laboratory by using the following tests: Anion 2- CO3 2- SO3 2SO4 Cl I - - NO3 Test Add dil HCl and pass the gas into lime water. Add dil HCl and warm. Test the gas with acidified potassium manganate (VII) paper. Add dil HCl. Then add barium chloride solution. Add dil HNO3. Then add silver nitrate solution. Add lead (II) nitrate solution. Add dil HNO3. Then add silver nitrate solution. Add lead (II) nitrate solution. Add dil NaOH solution. Then add a piece of aluminium foil. Warm the mixture. Test the gas liberated with moist red litmus paper. Observation and conclusion Effervescence is observed. The lime water turns milky. Carbon dioxide is liberated. The potassium manganate (VII) changes from purple to colourless. A white ppte of barium sulfate is formed. The ppte is insoluble in dil HCl. A white ppte of AgCl is formed. The ppte is insoluble in dil HNO3. A white ppte of lead (II) chloride is formed. A yellow ppt of AgI is formed. The ppte is insoluble in dil HNO3 A canary yellow ppte of lead (II) Iodide is formed. The red litmus paper turns blue. Ammonia gas is liberated. Chemical test for water. Pure water is colourless and odourless. There are two chemical tests which can be used to detect the presence of water. 1. Water changes the colour of anhydrous copper (II) sulfate from white to blue. CuSO4 (s) + 5 H2O (l) CuSO4.5H2O (s) Anhydrous Copper (II) sulfate Hydrated copper (II) sulfate (Blue) (white) 2. Water also changes the colour of dry cobalt (II) chloride paper from blue to pink. CoCl2 (s) + 6 H2O (l) CoCl2.6H2O (s) Anhydrous Cobalt (II) chloride (blue) Hydrated Cobalt (II) chloride (pink) Note: 3 The above tests can only be used to detect the presence of water. It cannot be used to detect the purity of water. Test for gases When a salt is being tested in the laboratory, a gas is often liberated. Gases can be identified by the following tests: Gas Hydrogen Colour & odour Colourless & odourless Oxygen Colourless & odourless Carbon dioxide Colourless & odourless Chlorine Greenishyellow gas with a pungent smell Sulfur dioxide Colourless gas with the smell of fire crackers Ammonia Colourless gas with a pungent smell Test Place a lighted splinter at the mouth of the testtube containing the gas. Insert a glowing splinter into the testtube. Bubble the gas through lime water. Place a piece of moist blue litmus paper at the mouth of the test-tube containing the gas. Place a piece of filter paper soaked in acidified potassium manganate (VII) paper. Place a piece of moist red litmus paper at the mouth of the test-tube containing the gas. 4 Observations The lighted splinter is extinguished with a 'pop' sound. The glowing splinter relights / rekindles. The lime water turns milky (a white ppte of calcium carbonate is formed) The blue litmus paper turns red and is then bleached (turns white). The potassium manganate (VII) paper changes from purple to colourless. The red litmus paper turns blue.