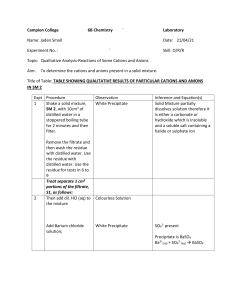
Qualitative Analysis Qualitative analysis is chemical analysis designed to identify the components of a substance or mixture, e.g. identifying the cations and anions in an ionic compound. Al3+ and Pb2+ have the same observations with both aqueous sodium hydroxide and aqueous ammonia. To distinguish between these two cations, add a few drops of potassium iodide (sodium iodide can also be used) to the solution which contains either of these two cations. If Pb2+ ion is present, a bright yellow precipitate will form as lead (II) iodide is formed. Equation: Pb2+(aq) + 2I-(aq) PbI2(s) If Al3+ is present, then, no precipitate will form. NB: If the solution contains K+, Na+ or NH+4 ions, no precipitate will form with aqueous sodium hydroxide or aqueous ammonia as these ions form soluble salts.