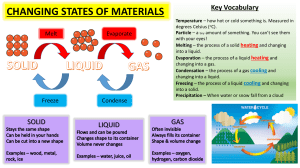
General Chemistry 2 Science, Technology, Engineering, and Mathematics Try It! Calculate the amount of energy needed to raise the temperature of 300 grams of water in the liquid state from 20 ºC to 27 ºC. 2 Lesson 2.3 Heating and Cooling Curves General Chemistry 2 Science, Technology, Engineering, and Mathematics When you take the ice out of the refrigerator, it starts to melt. 4 Water can be further heated in a kettle to boil, forming water vapor rushing out and mixing with the air. 5 As water changes phase, hydrogen bonds are broken, along with weaker intermolecular forces of attraction. 6 But how much heat is needed to transform ice into water vapor? Is there a way to represent these heat changes and interpret the energetics of phase changes more systematically? In this lesson, you will learn how to construct and interpret heating and cooling curves and calculate the associated heat changes at each step. 7 How do you interpret heating and cooling curves? 8 Energetics of Phase Changes: A Recall Changes in phases are accompanied by energy changes → molar enthalpies The phase changes and their energy 9 Energetics of Phase Changes: A Recall Phase changes only occur at specific temperatures and pressures ○ freezing of water: at 0 OC and 1 atm ○ evaporation of water: at 100 OC and 1 atm 10 Energetics of Phase Changes: A Recall For phase changes at constant P, where q= heat absorbed or released (J); n = amount of substance (moles), and ΔH =molar heat associated with phase change (J/mol). 11 Energetics of Phase Changes: A Recall For temperature changes, where q = heat absorbed or released, J; m =mass, g; c = specific heat, J/(g x OC-1); and ΔT = change in temperature. 12 Heating and Cooling Curves ● Transformations of substances in real life involve multiple phase changes and temperature changes. ● There are instances when a substance in its solid state is converted into its gaseous state. ● In between phase changes, additional heat is needed to satisfy temperature changes. 13 Heating and Cooling Curves The amount of heat in complex phase transformations can be tracked in heating and cooling curves. 14 Heating and Cooling Curves In these curves, the temperature (y-axis) is plotted against total heat changes (x-axis). 15 Heating and Cooling Curves This heating curve shows the heat associated with the transformation of 1 mol of ice initially set at – 25 ºC and 1 atm to steam at 125 ºC and 1 atm. 16 Heating and Cooling Curves How do we calculate the amount of heat associated with the entire process? 17 How can one calculate the heat associated with consecutive phase transformations? 18 Heating and Cooling Curves The amount of heat can be calculated by adding all heat absorbed from each phase and temperature change. 19 Heating and Cooling Curves The process can be divided as represented by the segments. 20 Heating and Cooling Curves Blue segments represent temperature changes without phase change, while red segments represent phase changes without temperature change. 21 Heating and Cooling Curves Segment AB Segment AB represents a change in temperature of ice from –25 ºC to 0 ºC. 22 Heating and Cooling Curves Segment BC The ice cube starts to melt from point B and ends with point C. 23 Heating and Cooling Curves Segment CD Segment CD represents a change in temperature of water from 0 ºC to 100 ºC. 24 Heating and Cooling Curves Segment DE Water starts to boil from point D and ends with point E. 25 Heating and Cooling Curves Segment EF Segment EF represents a change in temperature of steam from 100 ºC to 125 ºC. 26 Heating and Cooling Curves Total Heat Total heat is additive, from point A to F. What is the total heat of the phase change of ice to steam from point A to F? 27 Heating and Cooling Curves Constructing Heating Curves ● To create a heating curve, one must plot temperature (y-axis) against heat (x-axis). ● Processes, where heat is absorbed to increase temperature, are represented by lines slanted upright, while horizontal lines represent phase changes at a constant temperature. 28 Heating and Cooling Curves Constructing Cooling Curves ● Cooling curves are the exact reverse of heating curves. ● Processes that require the release of heat are represented by lines sloping downwards, while horizontal lines still represent phase changes. ● A cooling curve can be produced from the heating curve by reading the plot from the top right to bottom left. 29 Remember In heating curves, processes with temperature changes without phase change are represented by a line slanting upward, while horizontal lines represent processes with phase changes without temperature change. 30 How can you describe the cooling curve when steam at 150 ºC is transformed into –15 ºC? 31 Let’s Practice! Calculate the heat required to transform 5 grams of ice at -10 ºC to liquid water at 80 ºC. Use the following specific heats: cice = 2.108 J/(g ✕ ºC), cwater = 4.186 J/(g ✕ ºC), cvapor = 1.97 J/(g ✕ ºC). Water has a heat of fusion (ΔHfus) of 6 000 J/mol, and a heat of vaporization (ΔHvap) of 40 700 J/mol. 32 Let’s Practice! Calculate the heat required to transform 5 grams of ice at -10 ºC to liquid water at 80 ºC. Use the following specific heats: cice = 2.108 J/(g ✕ ºC), cwater = 4.186 J/(g ✕ ºC), cvapor = 1.97 J/(g ✕ ºC). Water has a heat of fusion (ΔHfus) of 6 000 J/mol, and a heat of vaporization (ΔHvap) of 40 700 J/mol. The total heat required to transform 5 grams of ice at -10 ºC to liquid water at 80 ºC is 3446.5 J. 33 Try It! Calculate the heat required to transform 15 grams of ice at -5 ºC to liquid water at 90 ºC. Use the following specific heats: cice = 2.108 J/(g ✕ ºC), cwater = 4.186 J/(g ✕ ºC), cvapor = 1.97 J/(g ✕ ºC). Water has a heat of fusion (ΔHfus) of 6000 J/mol, and a heat of vaporization (ΔHvap) of 40 700 J/mol. 34 Let’s Practice! Calculate the heat required to transform a gram of ice at -50 ºC to steam at 120 ºC. Use the following specific heats: cice = 2.108 J/(g ✕ ºC), cwater = 4.186 J/(g ✕ ºC), cvapor = 1.97 J/(g ✕ ºC). Water has a heat of fusion (ΔHfus) of 6 000 J/mol, and a heat of vaporization (ΔHvap) of 40 700 J/mol. 35 Let’s Practice! Calculate the heat required to transform a gram of ice at -50 ºC to steam at 120 ºC. Use the following specific heats: cice = 2.108 J/(g ✕ ºC), cwater = 4.186 J/(g ✕ ºC), cvapor = 1.97 J/(g ✕ ºC). Water has a heat of fusion (ΔHfus) of 6 000 J/mol, and a heat of vaporization (ΔHvap) of 40 700 J/mol. The total heat required to transform a gram of ice at -50 ºC to liquid water at 120 ºC is 3157.8 J. 36 Try It! Calculate the heat required to transform 10 grams of ice at -200 ºC to steam at 200 ºC. Use the following specific heats: cice = 2.108 J/(g ✕ ºC), cwater = 4.186 J/(g ✕ ºC), cvapor = 1.97 J/(g ✕ ºC). Water has a heat of fusion (ΔHfus) of 6 000 J/mol, and a heat of vaporization (ΔHvap) of 40 700 J/mol. 37 Let’s Practice! Calculate the heat required to transform a mole of ice that underwent the change from A to F described in the heating curve next slide. Use the following specific heats: cice = 2.108 J/(g ✕ ºC), cwater = 4.186 J/(g ✕ ºC), cvapor = 1.97 J/(g ✕ ºC). Water has a heat of fusion (ΔHfus) of 6 000 J/mol, and a heat of vaporization (ΔHvap) of 40 700 J/mol. 38 Let’s Practice! 39 Let’s Practice! Calculate the heat required to transform a mole of ice that underwent the change from A to F described in the heating curve. The total heat required is 56069.9 J. 40 Check Your Understanding Identify if the following statements are true or false. 1. When ice melts, the temperature remains at 0 ºC. 2. In a heating curve, the x-axis is temperature, in ºC, and the y-axis is heat added, in J. 3. Above 100 ºC, the relevant specific heat to be used is that of steam. 41 Check Your Understanding Use the cooling curve for a hypothetical substance Z shown to answer the questions that follow. 1. What is the boiling point of the hypothetical substance Z? 2. Which segment represents condensation? 3. How long does it take for gaseous Z to completely liquefy? 42 Let’s Sum It Up! ● Heating and cooling curves are used to track heat changes associated with complex phase transformations. ● A heating curve is produced when the temperature changes (y-axis) are plotted against heat changes (xaxis). ● A cooling curve can be constructed from a heating curve by reading the latter from top right to bottom left. 43 Key Formulas Concept Energy change without phase changes Formula where ● m is mass (in g); ● c is specific heat (in J/(g✕0C)), and ● ΔT is the change in temperature. Description Use this formula when the material undergoes temperature changes but not phase changes. 44 Key Formulas Concept Energy change during phase changes Formula where ● q is the amount of heat, ● n is the number of moles, and ● ΔH is the molar enthalpy of the specific process. Description Use this formula when the material undergoes phase changes but its temperature does not change. 45 Challenge Yourself In a heating curve, temperature (in ºC) is plotted against heat changes (in J). Changes in temperatures for a specific state of a substance are represented by slanted lines. What does the slope of these diagonal lines represent? Briefly explain its significance. 46 Bibliography Chang, Raymond, and Kenneth A. Goldsby. General Chemistry: The Essential Concepts. New York: McGraw-Hill, 2014. Handwerker, Mark J. Science Essentials. San Francisco, CA.: Jossey-Bass, 2005. Hawe, Alan, Dan Davies, Kendra McMahon, Lee Towler, Chris Collier, and Tonie Scott. Science 5–11: A Guide for Teachers. 2nd ed. New York, NY: David Fulton Publishers, 2009. Petrucci, Ralph H. General Chemistry: Principles and Modern Applications. Toronto, Ont.: Pearson Canada, 2011. Silberberg, Martin S. Principles of General Chemistry. New York: McGraw-Hill, 2013. 47