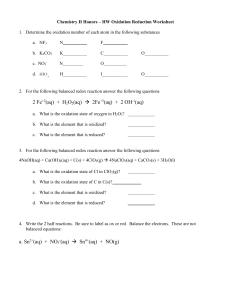
General Chemistry 2 Quarter 4 Module 5: Redox Reactions Prepared by: Jasmine D. Colcol Let’s review! What are atoms made of? • Protons • Electrons • Neutron Did I Lose or Gain? Decide whether the given half-reaction involves the lose or gain of electrons. Did I Lose or Gain? Li (s) Li + (aq) Did I Lose or Gain? S (s) S 2(aq) Did I Lose or Gain? Cr 3+ (aq) Cr 2+ (aq) Did I Lose or Gain? Cu Cu 2+ Did I Lose or Gain? Na Na 1+ General Chemistry 2 Quarter 4 Module 5: Redox Reactions Prepared by: Jasmine D. Colcol General Chemistry 2 Quarter 4 Redox Reactions Prepared by: Jasmine D. Colcol Learning Competencies: o Define oxidation-reduction reactions. (STEM_GC11AB-lvf-g-169) o Balance redox reactions using the change in oxidation number half-reaction method. (STEM_GC11AB-lvf-g-170) o Subtasks: o Assign oxidation states to elements o Recognize the importance of the lesson to life. Redox Reactions • Redox reactions stand for oxidation-reduction reactions. • Redox reactions describe all chemical reactions in which there is a net change in atomic charge Redox Reactions Reduction (charge decreases) Oxidation (charge increases) Redox Reactions Redox Reactions Oxidation Number It is a concept that helps in determining quickly whether the substance is undergoing oxidation or reduction. Other terms used to refer to oxidation number is valence or oxidation state. Oxidation states is the electrical charge assigned to an atom according to a prescribed set of rules. General Rules in Assigning Oxidation Number 1. The oxidation state of a free and uncombined element is zero. Li (s) N2 General Rules in Assigning Oxidation Number 2. For a monatomic ion: oxidation number = ion charge Cu 2+ General Rules in Assigning Oxidation Number 3. The sum of oxidation number values for the atoms in a molecule or formula unit of a compound equals to zero. (and equals to the ion’s charge if it is a polyatomic ion) H2O NH41+ General Rules in Assigning Oxidation Number The usual oxidation state of: a. Hydrogen is +1, except in metallic hydrides ( Li+1H-1 ) b. Oxygen is -2, except in peroxides where it is -1 ( H2+1O2 -1 ) c. Elements under group IA is +1 (Na+1) IIA is +2 (Mg+2) IIIA is +3 (Al+3) Know my State! Determine the oxidation number of each element in the ff: 1. CaO 2. KNO3 Know my State! Determine the oxidation number of each element in the ff: 3. H2O2 4. O3 Know my State! Determine the oxidation number of each element in the ff: 5. S2O7 -2 Let’s Practice! Identify the material being reduced, the material being oxidized, the reducing agent, and the oxidizing agent in each of the following redox reactions: 2Cs + Br2 → 2CsBr substance reduced: ____________ substance oxidized: ____________ oxidizing agent: ____________ reducing agent: ____________ Let’s Practice! Identify the material being reduced, the material being oxidized, the reducing agent, and the oxidizing agent in each of the following redox reactions: 2Sr + O2 → 2SrO substance reduced: ____________ substance oxidized: ____________ oxidizing agent: ____________ reducing agent: ____________ Let’s Practice! Identify the material being reduced, the material being oxidized, the reducing agent, and the oxidizing agent in each of the following redox reactions: Si + 2F2 → SiF4 substance reduced: ____________ substance oxidized: ____________ oxidizing agent: ____________ reducing agent: ____________ Redox Half-Reactions • Redox reactions can be broken up into oxidation & reduction half reactions. 2+ 2+ Pb (aq) + Zn(s) → Pb(s) + Zn (aq) Reduction Half-rx: Pb2+(aq) + 2e- → Pb(s) Oxidation Half-rx: Zn(s) → Zn2+ (aq) + 2e- Redox Half-Reactions • Redox reactions can be broken up into oxidation & reduction half reactions. 2+ 2+ Pb (aq) + Zn(s) → Pb(s) + Zn (aq) Reduction Half-rx: Pb2+(aq) + 2e- → Pb(s) Oxidation Half-rx: Zn(s) → Zn2+ (aq) + 2e- Redox Half-Reactions • Redox reactions can be broken up into oxidation & reduction half reactions. 2+ 2+ Pb (aq) + Zn(s) → Pb(s) + Zn (aq) Reduction Half-rx: Pb2+(aq) + 2e- → Pb(s) Oxidation Half-rx: Zn(s) → Zn2+ (aq) + 2e- Let’s Practice! Identify each of the following as examples of oxidation or reduction: ▪ Li(s) ▪ S(s) ▪ + → Li (aq) +e 2+ 2e → S 3+ Cr (aq) (aq) 2+ + e → Cr (aq)