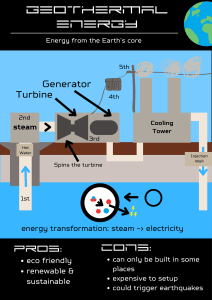
With Permission MECH 2202 Thermodynamics (W15) A01 Term Test #3 University of Manitoba Department of Mechanical Engineering MECH 2202 Thermodynamics (W15) Term Test #3 Page 1 of 2 (Prof. S.J. Ormiston) 13 March 2015 Duration: 100 minutes 1. You are permitted to use the textbook for the course and a calculator. All other electronic devices are not permitted. 2. Ask for clarification if any problem statement is unclear. 3. Clear, systematic solutions are required. Show your work (marks will be assigned for writing the equation, the substitution, and the final answer). Marks will not be assigned for solutions that require unreasonable effort for the marker to decipher. 4. Use linear interpolation in the property tables as necessary. 5. Keep 4 significant figures in intermediate calculations, and use 4 or 5 significant figures in final answers. Final answers must have units. 6. As appropriate, indicate clearly in your solution the boundaries of the control mass or control volume you are using to apply the laws of thermodynamics. 7. The weights of the two problems are given. The test will be marked out of 100. Solve both problems. You are not obliged to solve the problems in the order presented. Values 35 1. Consider the gas turbine system shown schematically in Figure 1. The system contains two stages of compression with an intercooler between the stages. Air enters the first stage at 100 [kPa] and 300 [K]. The pressure ratio across each compressor stage is 5:1 and each stage has an isentropic efficiency of 82%. Air exits the intercooler at 330 [K]. The maximum cycle temperature is 1500 [K] and the cycle has a single turbine stage with an isentropic efficiency of 86%. The cycle also includes a regenerator with an efficiency of 80%. Treat air as an ideal gas with constant specific heats. Assume negligible changes in kinetic and potential energy. 1 P1 = 100 [kPa] T1 = 300 [K] air T3 = 330 [K] P8 = 100 [kPa] 8 3 Regenerator Burner Intercooler C1 5 4 2 Q̇B C2 7 6 Ẇnet T Tmax = 1500 [K] Compressor 1 Compressor 2 Figure 1: Nomenclature used in Problem #1 Turbine 12 (a) Determine T2 and T4 and the specific work for both compressors. 13 (b) Determine the cycle thermal efficiency. (c) Show a process representation of this system on a T –s diagram. Show the states and the processes (use a dashed line if the path is unknown). Label state temperatures (except T8 ) and constant pressure lines. Draw your diagram carefully so that your states are shown correctly relative to each other (pay careful attention to the state temperatures). Do not label s values. 10 1 MECH 2202 Thermodynamics (W15) A01 Term Test #3 65 Page 2 of 2 2. The small power plant shown in Figure 2 uses water as the working fluid. Steam enters the first turbine stage at 3 [MPa] and 600 [◦ C] with a mass flow rate of 25 [kg/s] and expands to a pressure of 500 [kPa]. Some steam is extracted at 500 [kPa] and fed to an open feedwater heater (FWH); in addition, a steam supply of 5 kg/s is taken out and not returned. The missing water is added to the FWH from a supply at 20 [◦ C] and 500 [kPa]. Steam expands in the second stage of the turbine to the condenser pressure. Flow exits the condenser as saturated liquid at 45 [◦ C]. Assume all turbine stages and pumps operate isentropically. Assume negligible changes in kinetic and potential energy. P5 = 3 [MPa] T5 = 600 [◦ C] Q̇B 5 ṁA TS = Turbine Stage Boiler ẆT ṁA = 25 [kg/s] 4 Turbine FWH = feedwater heater TS1 P6 = 500 [kPa] Pump 2 TS2 6 6 ṁB water resupply ẆP 2 ṁR steam supply ṁR = 5 [kg/s] 8 Pump 1 T1 = 45 [◦ C] 500 [kPa] saturated liquid ṁC 7 Open FWH 3 6 Condenser 2 1 ẆP 1 saturated liquid Q̇C Figure 2: Nomenclature used in Problem #2 24 (a) Determine the specific enthalpy at all the states. 17 (b) Determine the power output of the turbine (including both stages), ẆT . 12 (c) Determine the cycle thermal efficiency. 12 (d) On a T –s diagram, draw a process representation for this power cycle. On the diagram, clearly indicate the labelled state points, the process paths (use a dashed line if the path is unknown), and the constant pressure lines that pass through the state points. Indicate all state temperature values except T2 and T4 (labelling them is optional – there are no marks allocated for determining their values and labelling them). Do any additional work necessary to label the diagram. Draw your diagram carefully so that your states are shown correctly relative to each other (pay careful attention to the state temperatures). Do not label s values. 2