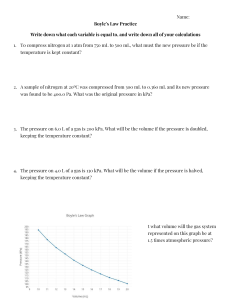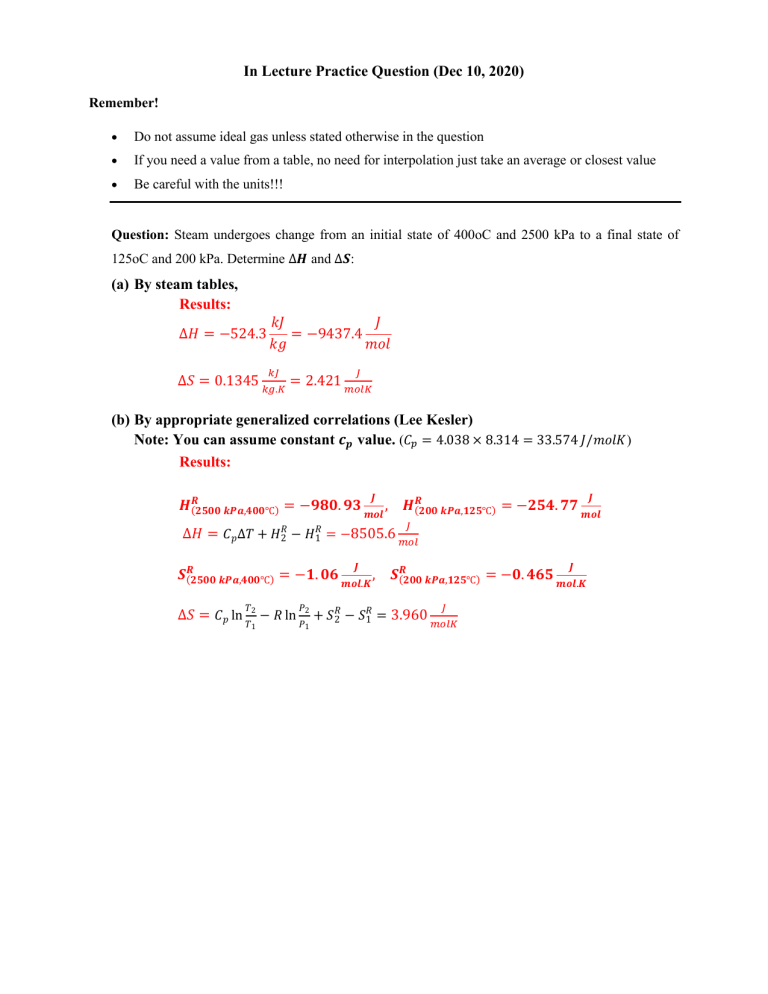
In Lecture Practice Question (Dec 10, 2020) Remember! Do not assume ideal gas unless stated otherwise in the question If you need a value from a table, no need for interpolation just take an average or closest value Be careful with the units!!! Question: Steam undergoes change from an initial state of 400oC and 2500 kPa to a final state of 125oC and 200 kPa. Determine ∆𝑯 and ∆𝑺: (a) By steam tables, Results: ∆𝐻 = −524.3 𝑘𝐽 𝐽 = −9437.4 𝑘𝑔 𝑚𝑜𝑙 𝑘𝐽 𝐽 ∆𝑆 = 0.1345 𝑘𝑔.𝐾 = 2.421 𝑚𝑜𝑙𝐾 (b) By appropriate generalized correlations (Lee Kesler) Note: You can assume constant 𝒄𝒑 value. (𝐶𝑝 = 4.038 × 8.314 = 33.574 𝐽/𝑚𝑜𝑙𝐾) Results: 𝑯𝑹(𝟐𝟓𝟎𝟎 𝒌𝑷𝒂,𝟒𝟎𝟎℃) = −𝟗𝟖𝟎. 𝟗𝟑 ∆𝐻 = 𝐶𝑝 ∆𝑇 + 𝐻𝑅2 − 𝐻𝑅1 𝑱 𝒎𝒐𝒍 , 𝑯𝑹(𝟐𝟎𝟎 𝒌𝑷𝒂,𝟏𝟐𝟓℃) = −𝟐𝟓𝟒. 𝟕𝟕 = −8505.6 𝑱 𝒎𝒐𝒍 𝐽 𝑚𝑜𝑙 𝑱 𝑱 𝑺𝑹(𝟐𝟓𝟎𝟎 𝒌𝑷𝒂,𝟒𝟎𝟎℃) = −𝟏. 𝟎𝟔 𝒎𝒐𝒍.𝑲 , 𝑺𝑹(𝟐𝟎𝟎 𝒌𝑷𝒂,𝟏𝟐𝟓℃) = −𝟎. 𝟒𝟔𝟓 𝒎𝒐𝒍.𝑲 𝑇 𝐽 𝑃 ∆𝑆 = 𝐶𝑝 ln 𝑇2 − 𝑅 ln 𝑃2 + 𝑆𝑅2 − 𝑆𝑅1 = 3.960 𝑚𝑜𝑙𝐾 1 1