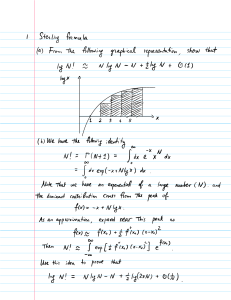
Organic Chemistry 26 Nov 2023 Old Concept Till 18thcentury it was believed that organic compounds are only synthesized in living organisms as they require some mysterious unknown x fories vital force for their preparation That is why organic compounds can not be synthesized in labs vital force concept organic organism living Modern Concept a Wohler's Experiment 1828 Ammonium Cyanite boil in water NA Tiffani NHz urea organic This experiment led to the failureof vital force ioniept organic compounds are hydrocarbons and their derivatives with the Cox carbon oxides 1032 carbonates HC03 exception of bicarbonates Naz103 Nak 103 H2 03 102 CO allinorganicsubstancet Compounds containing carbon t Hydrogen along with Érigite IIR I it Aldehyde Fe R OH alcohol R X e alkyl halide etc Naming standards in organic compounds a Common naming marshgas eg b IUPAC Cha methane marshy areas origins All single covalent bonds 1C Alkane eg fiefcoils common naming all no of carbon atoms area single covalent bonds IUPAC eg CH COOH Ethanoic Acid Coot Cit COOH alkanoic acids Acetic acid vinegar common name IUPAC Rules Longest continuous carbon chain is identified as the parent eg Citz chain cha CH z CH I H2 ly butane X x pentane parent chain the parent chain are numbered in such a way that carbon attached with a substituent branch gets the lowest possible number Carbon atoms in Hz CH 120 CH z Hz CH z Hz substituent branch F G Alkyl Hydrocarbons Alkyl Alkyl Alkane H O CHz eg misiane methyl C2175 ethyl 2176 ethane brands connected to 2ndcarbon methyl t pentane substituent If two or more identical substituents are attached to the parent chain then a prefix di tri tetra 2 3 4 added before their names to show the amount of identical substituents along with the numbers is Cit CI I CH CI cat TH 3 2 3 dimethyl butane t 3 ft't't H C 2,3 a y't lit cha Cit ditz City its thy g c Hz it ICH tri methyl hexane city 4ethyl 3 methyl heptane 212 dimethylpropane If two different alkylgroup are attached to parentchain then the preferred alkyl group is the one which is closer to the end eg Citz Cit citz Citta cha Cha It I 1 Hz Hz CH 5 ethyl 2 methyl heptane citz Cit Citz CI CI Cha CI I 1 Hz Hz CH If two different alkyl groups are attached to the same carbon from the end preferred alkyl is the smaller alkyl methyl ethyl ethyl propyl uts g't propyl butyl it if Hz 3 ethyl 2,4 dimethyl pentane Hz CH CH it it 2 methyl propane Hz C city I C H2 litz city Az t eh lit 2,2 5,5 tetramethyl hexane Alkenes Hydrocarbons with atleast one double bond term alk no of carbon atoms ene shows double covalent bond Additionally an appropriate number is added to the parent name to show the location of the double bond eg CHz CH ethene CH CH CH propene CHCHICI CI's 2 butene ou but2 ene Practice O Hz CH CH 4 methyl CHz II CH double bad is firstpreference nexene 4 methyl hex 2 ene CH EH CH CH CH CH CH Its 2,5 dimethyl 3 heptere 0 Hz CHCH Hz or CH 2,5 dimethylheat3 ene C CH CHEECH Hz 2,2 5,5 tetra methyl hex 3 ene CH3CHCHCH 2CH CH Clt CI CI e 6 methyl 42 hept L ene 1 Multiple double bonds compounds having more than one doublebond are named 2 double bonds alkadiene double bond alka tiene 3 4 double bonds as alka tetra ene alongside the name a position number is all o added to show clearly the position of the double bonds Practice CH z Chs CH CH CH CH CH 2,4 hexadiene It Git 115C 4 methyl CHz or hexa 2,4 diene CH Its 2,5 heptadiere or 4 methylhepta 2,5 diene CH CH CH It 3 CH Hz ditz CH 2,415 trimethyl t hexene or 3 4,5 trimethyl hex i ene y Alkynes hydrocarbons with atleast alk one triple bond of carbon atoms indicates presence of a triple bond shows no g ne A coefficient number is also added to locate the CHECH eg 43 C 2 ethyne C CH butyne bond CHz Ce c cha cha city 2 hexyne ou nex 2 yne Practice Citz CIC cha C't 9 II 8 citz City Cee II 5 methyl hex 2 yue C C CHz Clt It Ed Clt c'it gy EH É at a 5,6 dimethylhept 3 y ne Etz city 3 6,7 trimethylnon a yne 4 ethyl hex 2 y ne Compounds containing multiple triple bonds For two triple bonds alkadigne three triple bond alkatrigene For four triple bondi alkatekagere For Gt3 5 5 44 It c G methyl 2,4 heptadigne G methyl hepta 2,4 digne CH CEC CEC CE C C Hz 2,416 octatriyne Gt3 Cee II C C CHz Clt Clt2 4 ethyl 2,5 octadigne Hydrocarbons containing and bonds If or carbon bond is located at the same identified bond is then preferred C'Hz CH eg CH C Hz 2 hepten s y ne EE EH or hept 2 en f y ne bond is located at different carbon in the main chain then priority is given to the bond which is If or I closest to the end eg Cit 6 Ee ditz EH EH T 4 3 2 I hexen a y ne EH EH EH Cit Ei EH 2 a 2 6 3 hexer t y ne E Sunday, December 3, 2023 10:46 PM 5 I 2 3 a 6 dd 5methylnexzene Y'ts O ditz gg g a 314145 tetra ethyl 3 methylheptane methyldei 3en syne 2,2 9 9 tetra a ethyl 2,5 octadiane dodeca tri 2 Gloen4,8digre 2 3 dimethyl butz en e 2 2 3,3 tetramethylbutane n 2,2 33,4pentamethylpentane 2,5dimethylhex3 ene 2 s oc tadigne 2 3 6 7 tetramethyl 1 2,6 octadien a yne New Section 1 Page 1 Organic functional groups Chemical reactions Prep use application Functional Group Reactive part of the hydrocarbon chain Specie atom or a group of atoms that gives unique properties to the organic compound É or eg ester fruity smell OH alcohol Alkanes Hydrocarbons with all carbon carbonbonds orsingle covalent bonds Sigma bonds O CnHantz Bondangles 109.50 Shape Tetrahedral Nature of hybridisation sp Preparation of Alkenes Cracking Breakdown eg of a complex hydrocarbon into smaller hydrocarbons Cioltzo alkanet alkene t Types Catalytic thermal Hatley steam cracking A 203 Hydrogenation Addition eg of it to alkenes Jc ch alkene t ha Reaction Conditions s dd it it Ni Pt.PH CAIEAKo F.B P.B 200 3000C Ni 3001 SB MDCAT propene t Ha propane Sabatier Sander's Reaction Hydrogenation of alkane Oil h it Reduction of Alkane ghee parafins alkene industrial slate olefins Ayane saturated methane cannot bepreparedusingthismethod Decarboxylation of Sodium Alkanoate or decarboxylation ofsodium acetate KU decarboxylation of mono carboxylic acid delarboxylation of Sodium saltoffattyacid Two step reaction step1 E oh Naoh carboxylic acid caustic R Galkanoic a lid soda H2o R É on a sodium Alkanoate R É Ona t NaOH Step 2 Cao R H t Nao É Ona alkylth t Alkane Na 203 washingcode NaOH causticsoda City Clt Ggg mg off t soda lime Naoh É Ona t NaOH Y Hot cat Cha t É Ona sodium ethanoate Nazca methane É ona Nao CzHzÉ oh NaOH butanoicacid City citz Clf É Ona Nacht H2O t city cha cha É ons sodium b taroate Gt3 Citz Hz it propane Naza 2019Cmdcat Q Alkanes can beprepared by a dehydration of alcohol W b decarboxylation of sodium saltoffatty acid c hydrogenation of alkene a halogenation of alkenes From organo metallic compounds Grignard reagent R ng X alkyl magnesium halide By Hydrolysis through R ng x H ont t t R ng X alkane allyinghalide water X CL Br R it ng't't basicmg halide I Propane H ont t water If chloride c Ha ng CI cats propylmagnesium propane mg a basismagnesiumshlon eg H ont t Hz citz Mg Br EY City ethyl mg bromide Hz ethane Mg r basicmgbromide Reduction of alkyl halides R Antti citzCL In t Ha R H 2ns t 2nd z City Chemical Reactivity ofAlkanes In alkanes all C C bonds are sigma bonds ie electrons involved in bonding are under the influence of twonuclei So Chemical reactivity decreases as Bond Breaking requireshigh energy I Parafine 1 less reactive Free radicle substitution whatis a free radicle specie fanthate with an unpaired electron El neutral atom it neutral molecule They have highreactivity x How is a freeradicleformed Homolytic fission Heterolytic fission same atoms different atoms A U IB ITI Twofreeradicles A XB I A Bt anion catio Cla of City Initiation U Cla Iggy citti Plank's constant h f V new G 625 10 3 frequency of the photon Propagation radicle jethy'free ftp.jj Fie attack CIII CH di at a til HCL ICH2cL CHICK CHILI CHICK t CL HCL t CH CE CH Cl CHEL z t CL z Haz t CL C J see s CHL CL a Combustion Hy t 202 City Oz extess CO2 t 21 20 limit co H2o Oxidation Hy t I O 200atm methane CHsoit CO CH3OH s methanol s H EH H2o methanol HCHO CO HCOOH O Oxidation s Oxidation which can products I S HCOOH formicacid carboxylicacid CO2 H2O fully leads to combustion of a hydrocarbon producesorganic products further be oxidized to other organic Nitration R H CHL t HONG IT Honor É R NO H2O nitroalkane H2o CH t No nitromethane Sulfonation R H on Hy t 15 11 400 o it SO it Hot R so H alky sulphonic acid Hz 503A H2o methyl sulphonicalid Formation of Benzene Aromatization Cr O s 3CzH ethane IEE Cla and Bra Iz is Colts t GH Benzene proceed with free radicle mechanism a reversible reaction CHL t I ICH I HI Fluoro compounds compounds are prepared through Cly and Br CH CL F CH F t CE 8 KID of Alkenes Olefins s oil derivatives atleast one doublebond Sp Angle 1200 Shape Trigonal planar HW Physicalproperties uses from books across all provinces Reparation of Alkenes Dehydrohalogenation of allyl halides d from halogenoalkane removing H and X halide General equation I d I t II alcoholic a thot ice City Hach t Kot ka thro t CHz City ihloroethane ethene alkyl halide halogenoalkane Elimination reaction Saytzeff's rule it is removed from carbon adjalent to halide that has less H attached to it X R kcltHzo tkat 43 ftp g t at gicit sits Priority methyl but 2 ene major product 2 chloro 3 meth butane Prepare Cit 2,5 octadienefrom halogenoalkane It 1h2 Hz CH CH 2,5 chloro octane d 2K Clt 2720 CH CH City CH CH 2KOH CH TH CH CH Az woman wit 2,5 octadiene Dehydration of allohols d d I I 9 Hz 91203 388 HoH ethanol Aha 348L ice H2O Hao X Ha Citz ethene Ease of dehydration tertiary 30 alcohols mosteasily secondary primary 10 allohols 20 alcohols dehydrated eg city fits c 20 250 oh 2 methyl 2 Hz OH GH Hz I 850C It 2 propanol Is 2 propanol Hao Its methyl I propene l propene Holt ethan l ol C cha CH tho Hz Hz Hz it 140 C 1709 meat only 170 C H2 Ha t H2o ethene Dehalogenation a of dihalides halogen attained on two different carbons vicinal dihalides d d y f IUPAC t 1,2 y't s Jc d Intz alkene Zinc halide dihaloalkane vicinal dihalide Iz Iz eg 2h 2n At Cha Cha ethene 2nd zinc chloride dichloroethane vicinal dichloride 2019 AKU b halogens attached to geminal halides same carbons i I d It eg an a It feed anat Izz d Clt Incest CH I l dichloroethane CH CH CH but 2 ene a Prepare 2 hexene through geminal halide it d citz CH 22h t it is Geltz CH 22m42 É Hz CH CH CHCH CI hex 2 ene 27 Delember Lecture 11 Oxidation ofalkenes a Cold dilute Kmnog b Hot cone Amnon eg hydroxylation CH CH IUPAC t 103 Mnd CHz CA colddilute bit bit y ethan 1,2diol ethyleneglycol d oncommonname a ketone Jfc co no 2 Cat Hotions I Clt propanone 2,3 dimethyl 2 butene b Carboxylic acid c Hf t c CH cisbut2 ene o I 2citz É OH ethanoicacid HIIIII c c CO2 t H2O Mno Hz Cha t 4 o Its If af hey e CH hot gnc o 202 H2o 413 city Ifc city É opt ethanoicalid propanone o city I OH Coz Hao ethanoic acid Oxidationofalkenes with Agro H2 CHL A920 300 C Ha CH highpressure o O ethyleneepoxide epoxides are then used to prepare glycols in industries H2 CH CH 02 A920 H Clt 3000C highpressure City t n 102 propylene epoxide NCO t H2o 5 Combustion a ie b SC CI t c 6 Ozonolysis Oz 02 excess CO2 limited t co H2O H2o Reaction with Ozone 03 unstable d Hz cha t Hz Hz d O 03 rearmament o Structureisnotimportant CHIOTH d d 2n t H2O I 2HCHO Oy g Ozonide t H2O methanol H2O g 2nd t H2O Ozonolysis is used to locate the positionof double bonds 7 Addition D of hypohalous acid HOX X CL Br disproportionation Prep H2O HCL Lz HOLL I HO CI t cha cha hypo chlorous acid Ha GH Gtz chlorohydrin 2 chloro l ethanol IUPAC ethylene chlorohydrin F a H2o 7 Ho Br HB Bra Ha Cha t HoBr Cha Cha dit br Bromo hydrin 2 bromo l ethanol IUPAC ethylene bromohydrin F G blvd graduatedfrom Yappington University of yappers true 8 Addition of Hasou je c t production of alkyl hydrogensulfate I 440 5034 so H alkylhydrogen sulfate dd f f so t H OH 10 d IH 617 t it so alcohol eg Cha cha t it 0 50317 Hz CH 6 So H ethyl hydrogen sulfate CH Ha d 503A H2o 100 Hz CH OH ethanol t Hasan Markovnikov's addition is valid Iot xts is addedto carbon with less H its cats Hoke I Chace dits major product Lecture 12 9 Polymerization aku neitz it 2,88 m tracesofOz t.CH CHA polyethene Cont mdcat n CH Clt 200 sooo atm f CH CHI polyethene Good quality polymers are produced with Alkaits aluminum triethyl and Tilly TitaniumTrichloride 10 Mustard Gas 2GHz cha g 52cL t H2 CHz Ce cha cha a s mustard Gas chemicalweapon 12,2 dichloroethy sulfide Ithysical uses properties across all boards d Prepare Propanol from alkene Hal gift I Ifj 440 5034 Azl chicha t H oh 1009 gg alcohol t Hason Bromohydrin from alkene Ho Br Ha Cha ethene Cha Cha dit Br ethylene bonohydrin 2 bromoethan 1 ol 2 bromo 2 methyl propane CH3 City d city t ABR HzC Prepare vicinal dibromide from an JC CI t Bra Br 4h3 C CH b alkene e d d Br Br Prepare propylene epoxide CHz CH CH 02 A920 300C highprenue H2 CH CH o propylene epoxide Ozonolysis of propene city CH CH Oz 1 20 Formicacid from alkene CH I citz o HCHO methanol city I ethanol methodic acid HCHO Ketone from alkene CHz Copy previous notes pls CH CH CH it Hag Lecture 14 Alkynes hydrocarbons with at least one triple bond b w 2 carbon atoms general representation as general formula CnHan 2 Hybridization Shape linear sp s t as in C C two p orbitals in carbon are involved pi bonding angle 1800 alkenes alkynes alkanes highly unsaturated mostreactive least realtive General methods of preparation Denydrohalogenation of alkyl halides case 1 vicinal dihalide É 2kg dihalo alkane alcohol ethanol 100 C AKU J 200 C Noms IBO c 2K X t 242 Ot Cee eg Hz EY CH 2KCC 21120 2K017 CH Ce Ct but 2 y ne Hy GHz t 24017 910h ROH Kart H2o Bu Br GI Cha CHECHtKBrth vinylbromide 112 dibromoethane vicinal dibromide Sodium amide it it t amanita gg II's c c t 2 wax 2nits above reaction is used to form terminal alkynes l b tyne 43 g CHz CH 2nanit CHIH Cha Cha t 2Nacht 2MHz As colt is a stronger alkali than Nana which moves the triple bond from terminal carbons to non terminal carbons Cha CH CH Clt d d I b tyre acidic 240179 11 CH If rearrange CH in KOH HC C CHz Hz CH 2k Cl t 21720 CEC CH 2 butyne Hz cased Geminal halide ÉE If y t Nan it lianas 330 2Nax 2Nitz CEC alkyne acytelene City citz call g tananitz 2NaCl 2m13 Hz CI CH I methyl acetylene i propyne at citz I at Hz cha C CH that anno 1002 Look avant I 3 cliff CH CHz CECH NaCl 2N It Éogi Dehalogenation oftetrahaloalkanes II 22 s 22ns LIC t x x alkyne tetrahalide i i HE EH 22nd Zn Cee t From Potassium and Sodium salt CHOOK citron Choo EI 11 ego anion Potassium fumerate or Potassium maleate t 2kt anode CH ooo 11 at of CHIH 2102 the Industrial prep of acetylene Wohlers method Cao t 3C Calz 20 Cacz CO chm carbide 2420 i Ca OH t H CE CH acetylene Byshorter alkynes HC ICH anana 9Mt TEETH Tstrong 2Mt Tidicbehavior Nac Cwa ofethyne disodiumacetylide bond NAC E Cna t 2RX HC CH t 2Nana NAC CNG 2CH C S 2NaX t Nac IR CE c R Cna t 2Mt CHS LIC CH t 2Nall 2 butyne Lecture is Lecture 16 Addition of NH3 78 H NH Ca Hz rearrangement s methyl nitrite CN HC CH i I NHz ethane nitrile CHz Hz t Cy T CEN nitrile Addition of HCN hydrogen cyanide HC CH t HCN Chasin Nitigs neat HC I H CH I CN cyano alkene cyano ethene acrylonitrile C N Oxidation of ethyne a with acidified KMn04 CH CH HOH formicacid acetylene Hoot methanoicacid b With alkaline Amnon H C C Cit E Cit aietylene ethyne c y y H COOH COOH oxalic acid glyoxal CN ethandi oic acid oxaldehyde with 426207 42504 CH CH CHICOOH ethyne acetic acid vinegar ethanoic acid d Ozonolysis mdcat ICH 03 ozonide Ho s glyoxalt 172024 It 100 Polymerization CU 24C CH NHK CHz CH CECH vinylacetylene CHz CH CE CH CHz CH CECH t HC CH HCL conc s CHz CH CIC CH CH diving acetylene chloropopane CH CH C CH L Chloroprene neoprene This is used in industry to formsyntheticrubber Formation of Benzene 3ACICH 4 451 Colts I Acidic behavior ofethyne DistinguishingTests reddishbrown dicopperaretelide HC CH t CuzCuz Cu CE Ccu 2nAGOH n ethyne donates proton IN HULL t 2h20 acidic nature HC CH t 2Agnost 2NHaOH AgCICAg d 2N 4 NO t H2O disilveracetylide white pt Combustion ethyne 02 coz water physical properties t uses t C N Benzene Belongs to cyclic conjugated planar hydrocarbon family All hydrocarbons that include benzenering are classified as aromatic Aromatics Monocyclic of one benzenering consisting phenol Polycyclic ofmultiplebenzenering composed biphenyl qty Common monocyclic benzenoids II If methylbenzene toluene halogen phenol It Anilineaminobenzene If benzoic acid benzaldehyde O É II halobenzene benzene sulphuric acid IF IF nitrobenzene trinitrotoluene 219,6 trinitrotoluene TNT Polycyclic Fused Benzene Isolated BenzeneRing ring Isolated Benzene Rings bi phenyl EY 8 diphenyl methane j Mmds bakelite It Fuzed Benzene Rings naphthalene Naming Benzenoids It chlorobenzene anthracene phenanthrene C't É bromobenzene ethylbenzene Before After Fluoro carboxylic acids chloro Benzene esters carboxylategroups Bromo Sulfonic acid Todo Aldehyde methyl only nitro No a Ordeofnaming TopHighpriority 5 OH soon 2 CN 1 6 Nitz 3 CHO 4 COCH3 I 71 OR 8 R Cito 2 aminobenzaldehyde II 3 hydroxybenzoic acid IHO II Etna 3 aminobenzaldehyde É 4 methylphenol IIe on 3 methylbenzoicacid The Cito 3 ethylbenzaldehyde I B alphabetically naming all those aromaticswhich are notpresentin the priority list 3 chloro do benzene 2 Bromonitrobenzene If 4 Chlorofluorobenzene Eddo 2 chloronitrobenzene II 4 chlorobenzene s lfonicacid I I 2 iodo toluene na iii 3 Sulfonatebenzoic acid Eden 3 nihocyanobenzene 3 nitrobenzene cyanide ch eg R chlorotovene O Chlorotolvene same shit III Be p bromobersoicacid lectures I m Bff it 2,6 dibromophenol methyltoluene II 1,2 3 m methylphenol EI 1,2 4,5 tetramethylbenzene p methyltoluene at o methylphenol Epa h orthopara dichloroaniline No tribuomobenzene o It IF I so at NOz TNT 2,4 6 trinitrotoluene PH Eton III durene Resorcinol Ctyych3 Its structure mesitylene of Benzene Colts El resonance inspired EI hybrid by it Keller C Hi A of kekules structure Higgs H dream of 1865 Sigma bonds pi each carbon is sp hybridized nature sp 1200 angle bonds 12 317 715 9 g Shape Trigonal planar all bond angles either c c c or C H C Kmndc alkaline are is 1200 in benzene NOT decolorized in benzene showing benzenes unsaturated nature Similarly benzene show both addition and substitution reaction Addition Ha 4 t z s benzene ring is terminated It2509 HN03 Substitution Benzenes characteristic chemical reactions are substitution reactions and not addition reactions Preemie of 3T bonds alternating b w carbon atoms prevents H 8 if IA benzene the A ring to show addition H q H 8 H if f it 8H H Et IRayanalyeof c 1.3970A c Bee or 10 m 397 bond length 109A C H IA C degreeangstrom C C 1.397 x 10 10m 1210 12 1.627 10 27 Kg Comparing Benzene with 1,3 5 hexatiene cyclic 1 3 double bonds 3,5 cyclo hexahiene 3 double bonds Cyclic bond enthalpies for reduction t II H2 I 119.5KMmol 2172 I 2375kt mo I FI t 342 II 3172 Eye less y I 358.5452mA 208kt mol 1135,5ft I I Bok J not Benzene is more stable saturated than 1,3 s cyclonexatrien hypothetical Lecture 19 Benzene does not show oxidation reactions with Kmnoz and Racroz ie it doesn't decolorize themas for alkenes and alkynes This shows that benzene is not as unsaturated as otherhydrocarbon with double or triple bonds Benzene shows addition reactions to hydrogen and halogens But it shows substitution reactions with Hzson and H NO This concludes that benzene is not as saturated as alkane altane amine alkyne benzene lesscaturated more saturated Benzene was first isolated by Faraday in 1825 through distillation in 1845 isolated by Hoffman through coal tar Bituminous coal 1000kg of vegetable oil coal tar hat Benzene 1kg wog Structure of benzene Ey It deleted structure 1865 E inspired by dream Other structures EI Claus's structure ED Dewar structure ET I FI Baeyer's and Armstrong structure In Aromaticity Test Huckel Rule All aromatic compounds should have Lint 2 IT To f no of electrons electrons in benzene IT bonds in benzene Lintz I if n is a Lint 2 Lin a it 6 3 electron whole number aromatic compound 6 aromatic compound Cyclohexane telethon 2 IT bond 2 ant 2 2 4n 0 Questions Lintz a n Yz X Lin n 2 312 8 X I 5 Comparison of C C bond Alkanet 15h A Alkenes 1.34 A Alkyney 1.20 Eiti 1 397 A of Benzene Preparation Dehydration of cyclohexane I Ed t 3172 Éagm From Acetylene 3 HE CH organo Ni catalyst at 70 From alkanes 9 b Hexane 8203 41203 5p 4h 5009 442 I Heptane Toluene Laboratory preparation from sodium salt na t KI sodium benzoate of benzoic acid and soda line NaFEII narco By distillation of phenol É 2n t Zno t It By hydrolysis of Benzene sulfonic acid fig H at steam Has04 Wurtz Fittig Reaction Ei Ey r x t.IE alkyl eg 1 2ha tana t Clt bromobenzene Brt 2N a ether Bethany methyl benzene Through polymerisation 3ethyne Cu tube 500 C red hot 2nabr s benzene Benzene is BP a colorless liquid 80.12 insoluble in Physical properties water H 4 Ca 44 CaAz Lecture 20_ Chemical reactions of benzene Benzene performs electrophilic substitution reaction to IT to temp 255 EEF pop conc acids M dat it no 50 C c EI at anMÉÉtshould be less than 55 Aku Num Fedten Jotos H No It so It nitric sulfuricacid lewis acid lewis bale bronstead lowrybase brownitedlong acid H2o Noatt Hson EoY at Electrophile N 02 nitronium ion nitryl cation nilroniumionlnitryicati ppn.IE Arenium ion O NE e ont o t o election withdrawing eletchon withdrawing Step 2 two Hson Ew t Hasan Ey EF EFE repulsion is causeddue to eaten at If temp exceeds 55 then furthersubstitution take place t oh 24h03 I 708 no t 2h20 1,3 dinitrobenzene s I raw chemical forparacetamol I H Other substituents TNT NY na TNB Nos no N Picric a lid No Nor Noz 2 Sulfonation Etat Hag Q temp acid H2o Benzene sulfonic acid 809 Federal Punjab Allo El 3H I HII t 4420 forsulfonation in the presence of oleum fuming s ifuric 17250h 503 425207 gyro s lforic oleum temp 259 Electrophile for sulfonation is 503 g SO H O s at JI ÉÉt HEE FT't I É o H505 It so t k so Ht o 03h Hzsont benzene sulfonicacid Halogenation Al Xz catalyst F or Fetz reaction too Vigorous Is reversible poor yield It Xt electrophile 4T Halogeniunion formation of electrophile electrodeficient Step 1 X2 XX n N Fetz 4 Xt ou feta tetra halo ferrate ion 1 1 4 I fexj 3 Creek Ferroustate Attackof Xt EFI Txt carbocation intermediate areniumrion FEI IEEE EEE lil I Resonancestructureit Recovery of catalyst IF Feta HX fex t t II ILxjhaiobe.se Br Es X2 room try Al Bri EFI EEE ETB Allu Num II r 259 I preevre Alkylation and Acylation 1871 Charles FriedalfidIMetts Reagent R Catalyst Alt Electrophile Rt AIX X J RTX alkyllation ET't Rt Acyl cation Eat Arenium ion Cracestuche ETI Rec IFI name rt Aix Ek Itr of catalyst t Alxi Htt Alxst ETR alkyl benzene Lecture 26 States of matter 5 8 Mcd's Gases Kinetic molecular theory Basic assumptions Gases consist of either molecules or atoms dependingupon the nature of the gas Henegar omitted Individual molecular volume of gas molecules is considerednegligible Gas molecules perform translator motionwith no loss in energy Gas molecules only exert pressure when they collide with the walls of the container RE of gas molecules is mainly associated withthe translator motion Distante travelled a by ga moleales before collision is freepath gas present in as compared to a a large container will have greaterfreepath a small container gas in O O freepath to hision free There are no intermolecular forcesofattraction or repulsion b w gas molecules Gas molecules can be considered an elasticrigid spheres ideal gas imaginary gas obeys all postulates ofR m t at all conditions of temp andpressure Any gas behaves ideally at high tempand low pressure Rudolf Clausius 1817 All gases that exist are real gases andthey do not obey K M T KE n T t absolute temp Kelvinscale KE or translatory Int Ime I average velocity A Ideal gas will behave H T and L P b L T and H P a Lecture KE K K KE D c non ideally at at all temp andpressure d none of the above 27 3 KT Boltzmann's constant By 1.38 10 23 Jmol K f gas pressure average translational K E tmnt or Im NI Y C Vrms N numberofmolecules ang square velocity É IMY 3ft My 3kt x 3ft in Ft 31mF F 35nF K Fa k small ems Yams P PI p density Boltzmann'sconstant Ey Ft Gas Laws Defined on the basis of state variables State variables Parameters through which a state of a explained completely P s Y Pascals I atm t n Nmt atm non SI gas can be torr bar mm of Hg 101325 Pascals lat m 1 01 latm 1 101325 Pascals I torr 105 Pascal 105 Pascals 760 torr 133.33 Pascals 760mm of Hg Volume m3 SI unit Cms SI units non dm ml L Im 10dm Im 100cm Im 103dm Im 106am Im 103 L Im 106mL IL 1000mL Temperature SI K C non SI TK TF T C g Toc of 273 32 Tc tr 32 Kelvin Absolute Scale III I Farenheit s 32 F 212 F I'epoint Stearpont Fdi 100 dirt Ok 2 100div 1009 Absolute scale The smallest value in Kelvin Seale is 10C 1.8 F oc of 99 12,2oz 409 400 F TOF TOC E 32 O In a closed system no change in mall system Anything consideration open closed mass energy energy isolated no energyandmass Boyles Law Pat Isothermal Process P is constant K PY K Pill i temp Paltz for different states Pan hyperbolic i rate isotherm P P2P Ph BoylesLaw 4,1124344 p Y at a Kelvin constant Yy at constant pressure Isobaric Process n a T K Isochoric Process Volume is constant P RST 4 4 pi 273.162 TC d The ratio b w V and T at constant pressureis always a constant represents which of these laws a Boyle's b Charles c pressure law d none of these Avogadros Law Van at constant temp and pressure K y a n combining Boyles Charles P LII P const Ty R 8.313 Jmot K Re 0.0821 atm dm not K l Ptatm d what will be the volume of a height the where its original value prawn at Pay 3 time if it risen to 3rd of a balloon becomes torstar temp Lecture 29 Compressibility factor TimidEamat conditi n z any Pile n RT R z g PI R 2,42173K s idealgag p If diatomic gases at low pressure behaves ideally and high temp Two faulty assumption of K MT Individual correction molecular volume is negligible it is not negligible No attractive repulsiveforces b w gasmolecules correction gas molecules have weak attractive repulsiveforces Pkn RT invalid Vanderwall's correction of generalgas equation a P P tan pressure correction factor b K W tan ang a a nb x nb for pressure for volume volume correction factor n RT atm atm a Nff atmm.fm a wrists ann.mg a Nm G mo 2 V b nb dm b b dm did be my b Ly b n'n Diffusion movement flow of molecules from higherconc to tow cons Rate of diffusion rg re rs unit J I Ed y Effusion movement flow of molecules from higherconc to low cons that is comparable to the size through an opening hole of the molecules Graham's Law r tf of Diffusion or r a fa 12 NI TH z VN 2 Mr 2 ME 28 rate of diffusion of a gas is inversely proportional to the sq root of its molar mass or density Em eg E EEE Dalton's Law Pressure Partial of Partial pressure Ypresian of life widgeon 2atmtactm i d Any 1 Planet Ii 1 105 1.5 14 7 RT 8.31 iii d in b Db Vi 10 NXT xiii ft b ik R O 082m01 K atmdm Chemical Bonding Aku weigtage 2 3 mdcat q's 5 7g's Chemical bond Force of attraction btw 2 atoms holding them together in a molecule formed due to exceeding interatomic attraction into election repulsion T no attractive repulsive forces nuclear attraction on e attractive forces repulsive forces stronger weaker 7hpm bond length 75 4pm H H 17219 DH Higt Hg 436kt mol Hz molecule is formed Atomic Radius The distance b w centre of nucleus to valence shell A IA unit Group 10 m Down the new group a r increases shells added y Period s Across period a r decreases nuclear charge hold on valerie e decreases Radius Ionic Cationic Smaller than cationic Anionic larger than a r a r atomic radius radius t electrons less nuclear attraction on electrons nuclear attraction on more Valerie elections stronger valence electrons weaker divalent cation trivalent cation monovalent cation largest i r monovalent anion smallest i r anionic radius s smallest i r c divalent anion trivalent anion s largest i r Inter ionic distance anionic rt R radius r cationicradius eg KC rt Kt F Ch R 144pm do not learn covalent Radi half of distance b w 2 identical covalently bonded nuclei or half the bond length K H X H Hz molecule 7421 pm 37.25 pm Ionization Energy ell Joules minimum amount energy required to remove of 1 mole of e from gaseous atoms in the ground state Mg no s Mgt t e Ist I E state symbols Group Trend GT the group I E decreases as atomic size increases and nuclear pull on valence e decreases Down Period Trend PT Across period I E increases as atomic size decreases and nuclear pull becomes stronger Factors Atomic size Nuclear pull Shieldingeffect Type of orbital Electronegativity Relative tendency of an atom to attract a shared pair of electrons towards itself in a molecule F 4.0 e n Pauling's scale for e n has no unit F O s N CL N of H hydrogenbonding legit me Group Trend Down Br GT the group increases en decreases as atomic and nuclear pull on valence e Period Trend size decreases PT Across period e n increases as atomic size decrease and nuclear pull becomes stronger Nature of bonding can be predicted based on the different e in en DE N Type of Bond On 0.4 R alert non pop Impure covalent polar 0.4 n 1.7 17 Ionic d id Electron Affinity is added to 2 mole of gaseous atoms in their valene shell Energy change when I mole of e E N E A GT Decreases For For same trends PT metals metals the non Increases ve factors Theories of bonding Lewis concept of a chemical bond chemical bond is formed due to transfer sharing of e without any reference to atomic orbitals geometry of molecule interactions energy Ionic Bond electrovalent bond or non directional bond electrostatic attractions between cations and anion Not Na NaCl compound is 1007 ionic in nature Nacl 72 ionic in nature CSF is 921 ionic in nature no Covalent bond Bond that is formed due sharing of electrons to mutual equal Types Based on e n differences Polar covalent Impure covalent HCl NH3 HF H2O Non polar covalent pure covalents CO2 Based CH 4 on CCL4 number of election pairs pair of elections single covalent bond a I b 2 pairs of electrons double covalent bond Dative Bond covalent bond that is formed due to one sided sharing of electrons H I E N H H According to lewis concept covalent bonds are treated equivalent to dative bonds Eg H3 0 H o H Hydronium ion ftp.t Ht s ft ft or yet ft us bond in 7130 is treated as 33.331 dative each and 66.661 covalent NHation each R dative and 757 covalent Ion OH and OHtit oxonium R É H I bond in NHat is 251 Oxonium R H Ht Hsn Etat RER É H r ion with Ht 33.337 dative 66.661 covalent r E r Electron AffinityException Cla has higher electron affinity than Fz Smaller size of fluorine Incoming e repelled to greater extent Energy released in fluorine atom after gaining election slightly less than in chlorine First electron affinity is not always negative For metals first E A is positive as we need to supply energy to add an electron to metals Metals Non s metals Limitations to I the me I II V 117 1111 Lewis concept Only explains bond formation on basis of sharing transfer of electrons without explanation of atomic orbitals shape of molecule after bonding and energy interactions USE PR Shell Valence Electron Pair Repulsion Theory Nyholm and Gillespie bond pair Sedgewick and Powell 1940 lone pair Learn Shapesandbondangles Basic assumptions of USPER molecule is explained on the basis number of bond pairs and lone pairs a Shape of of a central atom has Election pairs in the central atom adjust in repulsion order minimum repulsion is between two lone pairs minimum repulsion is between two bond pains Maximum and one pair lone pair lone pair bondpair bond pair bondpair USPER Theory all bond pairs whether from single bonds or double bonds or triplebonds counts 1 According to Limitations of US PER overall geometry of molecule based on of e pairs in central atom Explains no orbitals involved in bonding and type of overlapping btw them Does not explain atomic Valence Bond Theory Heitter and London Main postulates is Is My H H H Is E Is 0 s Hz molecule orbital overlapped orbitals 2 S Sigma bond Atomic orbitals involved in bonding must have electrons in the opposite spin Number of bonds that are formed in a molecule equals the number of unpaired electrons in any one of the atomic orbitals p p Pl The atomic orbitals that overlap first forms a Sigma bond and orbitals overlapping after the formation of sigma bond always overlap sideways forming a IT bond Only those orbitals overlap which share the same symmetry along the bonding axis Energy is released when two atomic orbitals overlap the strength of the overlap determine the amount of energy released stronger overlap higher energy Op P highestenergy sign II lowest energy S S S S o bond S p bond Is s annan Pu Erin at Pn a Pn a Pn sigma overlap strongestsigma 84 I I 188 1 1 jpg 808 y y IT electrons o electrons it electrons Limitations of NBT atomic orbitals maintain their atomic identity even after forming a bond or they overlap No reference to atomic orbitals ofmolecule VBT is rejected Hybridization Mixing of orbitals ofdifferentshapes and energies to form orbitals of equal number and equalenergy C Is 2s Z D n p low energy J p p highenergy Spt Tetrahedral D eg city H g it 109.5 Sigma Dd bonds Conditions for not taking part in hybridization Empty atomic orbital Empty orbital involved in pi bonding H2O 0 8 152252274 Iif E H O B Dmr o H o BFz B 152 2s 292 D d dot tempty CO2 152 2s O 2,2 CEO DIII unhybridized orbitals Beclz Be 4 152s 2p IDB Molecular Orbital Theory Formation of molecular orbitals Two atomic orbitals can combine to form two molecular orbitals always Antibonding molecular orbital QQ high energy 2 atomic orbitals energy at low energy molecule orbital t Bonding molecule orbital Hz molecule I H Ish energy electrons y enter low energy orbitals D D is b Des k is two atomic 1st mo 01s s s ze se electronic config g Aufbau principle electrons occupy lowenergyorbitals first Bond order number of electrons in b m o numberofelectrons in a b m o 2 for Hz 22 0 1 for Hea He 2 152 1 11 n energy É 01s A single covalent bond Hz is possible 31 E o Hes not possible For Li 3 Li Is valence orbital 2s D a inside 0 25 251 251 a 0252 Bond order 2 1 21 Liz possible 015220 1522 02s B 5 1522522g n tu 0 252 pl 252 252 p 0252 gu 0 192 energy pl 152 pl I I 0152 I Pl 152 015220 1520252025 a 2p IÉzpn PHI energy I Een D T2py 2pm Bond Order 2 21 1 Bz is possible Paramagnetic due to unpaired e 0752 0 1520252 0 252 IZ PI Tay n C Cz hypothetical 15425 same ar B 1525292 Eal c e IT Zpy IT Pn Pn Py p p Pa Pa p Pg p Pz And Pr Pl ITZpy 1242 B O 4 21 double covalent bond 2 not paramagnetic as no unpaired é N Nz s 15225293 A N Fan IIpy energy 2 ee 21 2pm 227222 22 222 I T2py 6 7292 e e set B 0 N Ika de I 4272 3 Nz is a triple bond Na is possible Not paramagnetic no unpaired elections 0152C 0 1522025220 252 L IT2pf 2pm's 0292 Paramagnetic substances substances that are non ferrous and still attracted to external magnetic field neither attracted nor repelled Diamagnetic Oz is exception an O 152252274 Epn a I 2py unpaired e inmolewio 72,2 orbital fy y energy 2pm 22g 222 Get 01820 152 Bo p 0 63 exception due to high It yelectron pairrepulsion 28 OPALA2Py2 42 2 IT pact 2ply H 2pm double bond Fz molecule 1522522ps F En T energy I I y É2 N I Pl P In Ey Iz 2pm 22g 222 Y In B O 6 1 0152 0 15 Co 28 21 single covalent bond 0 282 02pm ITZpy IT 2Pa L IT 2pyZ IT 2pn2 non paramagnetic no unpaired e in molester orbital Bond Enthalpy Bond Energy Energy required to break single covalent bond Energy released when single covalent bond is formed Factors Bond order B Or B E AE Na B E Electronegativity eg Hah Help BL d Bond length Size of atoms EE A's at Dipole moment 2 money dipole charge on molecule qf distance b w charged molecules bondlength q le 1.6 10 19C Debye 3.3 10 I Debye 1 3 3 10 30 30 Coulomb meter m net dipoles O 0 Mnet O is met to p Wnet Bt f List of common values of dipole moment 0 Boh're atomic model 1911 Electrons revolve around nucleus in fixed energy shells levels orbits stays constant as long as it is in any fixed energy state orbit energy level Energy e Electrons absorb energy only to transition to high energy state excitation Electrons release energy when they transition to low de excitation energy ground state Absorption of Energy F ex E high t excitationenergy Ez E not bindingenergyfrom lower state Isbinding energy foot higher state E S E z Ez SEG E z Derivation only learn concept values formulas Radius of nth shell orbit of H atom Eres pgle Fes Electrostatic Centripetal Fc permitivity At ground equilibrium state of Fc FES MI ate free spare 9 atomic no MI Effort my Iffy only those orbitals are possible where angular momentum mxvxr n If th mur n no nd h of orbits3 6 625 10 T.se ZIT Sfm EE mffm MEE Ifor II Last Enter time im writing 25d ays r r e n't n'x on this ipad for 4k22 3 5 Sec x 8 825 10 12 c Nm 625 10 3 74 x 9.1 10 21 kg x I I 6 10 19C 10 10m n 2x 0.529 r no 529 A radius ra Energy of an e T E o or rs c ra ra cra r increases in nth orbit of H atom E K E P E Ifor xy Im at ME ate 3 x2 motion Emi EI PE I IN work done work F S jet r If x2 E PE K E EE CEE 21 En SEE r e En n II E_ EYE FELIN En atom Bohr's radius I f En constant 2.1681 10 J atom mole En 131,3 35 KT Mol J for H atom F ex Eh exi hf Ez ht 2 ht E E 1,68 2 70 410 18 18 2.168 10 I Rh fix 2.144 18 18 Fa Ie tint s Rh Rydberg's constant 1 09 107 E 13,31 Ez 1321 E3 13321 13.6 ex 3 4 ex 1 51 ex Atomic spectrum d group of wavelengths absorption emission excitation spectrum de excitation spectrum d s E neat neon 7 v light sourie go F I E f R ve Hz emission spectrum 7 6 5 4 I 3 2 E E E I E Ve Ve 1 Groundstat Lyman series 1 min may 1 09 107 1 I ultraviolet region 09 1 2 A may Eemin