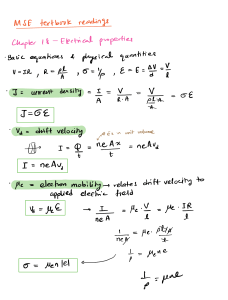
Valence Shell Electrons Electron Repulsion Theory USER repel each other Valence é pairs will arrange such that they minimize their mutual repulsion maximize the distance from each other Predicting VSEPR 1 Stene of lone pair t bonding pairs attached to central atom 2 Electron pair geometry 3 Molecular geometry I consider the atoms that are central atom Deriving VSEPR Theory Molecular Shape Elecotrons will situate in 3D Space so that they are not close to each other Consider the geometry about a central atom Steric SNI of central atom which is equal to of atomsbonded Thisgives the overall shape lonepairs t E P G ofthe of molecule ble both egoism at consider everything on central atom bonded to the Two types l Electron of geometries pair Geometry Relative positions of all lone pairs bonding pairs attached to the central atom EPG should always be one of the 5 2 Molecular Geometry common shapes of molecules Position of atoms bonds your molecules bonded to Central atoms Polarity E of dipoles in Dipoles vectors that lie of across bono t show the distribution of e E of dipoles polarity Polarity how are molecule behaves in a magnetic field It molecule aligns polar Polarity E of dipoles drawn for bonds in molecules t it polar have a permanent dipole left over from adding together dipole moments Shortcut If youhave unbalanced lone pairs Molecule Valence Bond Theory Explains molecular bonding Hybrid Polar Orbitals at the atomic level Premise A bond is formed when orbitals Overlap Ex H2 Mis t I n ti ti B o HF His His f f M I Hybridization overlap Bond 11 Yes H Fister n estetra a mixing of atomic new bonding sets called of lobes of orbitals need to hybridize to Cases where not Ex to make hybrid orbidals shape geometry tells us how The steric In between orbitals we make the geometry d bond internuclear axis orbitals need all many orbitals to be hybridized Haq a p C H 11 17 LS Oif p Mtsspzsf Ozo foxtails H I c c u 2P is l D off Ji 2mi is is lie Titi is is I SP is is r Is is Is r 088880 Shortcut SN For Hybridization of hybrid SN 2 SN 3 SN 4 SN 5 SNIG Additionally orbit as Ex SP SP É Sp SP'd I Spd for I single bond Acrolein M o O bonds M Abonus L 9AM centralatoms are SN 3 which H Means SP tf bonds 10 bond I Double Bond lo t t t bond I Triple Bono lot 21T bonds is