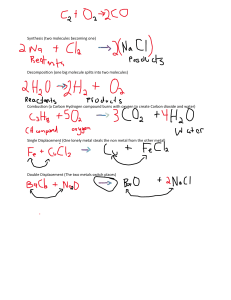
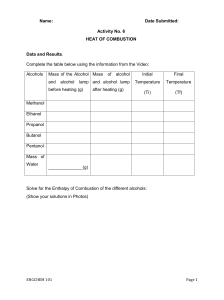
Theory: The combustion of organic compounds like alcohols produces large quantities of energy. Ethanol is a commonly used fuel in motor cars and its usage is increasing because it is a form of renewable energy. But what makes a good fuel? A good fuel is any substance which gives out large amounts of energy when it is burnt. In most cases, fuels are burnt in oxygen (air), i.e., they are oxidized. Is there any relationship between the energy released by one alcohol and another alcohol? This experiment aims to investigate the relationship between the number of carbon atoms in an alcohol chain and its standard enthalpy change of combustion. The heat of combustion (standard enthalpy change of combustion) is the enthalpy change when one mole of the compound undergoes complete combustion in excess oxygen under standard conditions. It is given the symbol ΔH˚ comb and standard conditions simply refer to room conditions with a temperature of 298K and a pressure of 1 atm. The combustion of alcohol is an exothermic process. It releases heat to the surrounding resulting to a negative value. Procedure: NOTE: Alcohols are organic substances that are flammable and easily catch fire when exposed to naked flames. It is a fire hazard. Care must be taken to ensure that any spills are being cleaned up immediately. Safety goggles must be worn while performing this experiment. A fire extinguisher should also be kept close by in case of emergencies. Theory: Calorimetry is the science of measuring a quantity of heat. Heat is a form of energy associated with the motion of atoms or molecules of a substance. Heat, Q, is measured in energy units such as joules (J) or calories (cal). Temperature, T, is measured in degrees Celsius,°C. Temperature and heat are related to each other by the specific heat, cp, of a substance, defined as the quantity of heat needed to raise the temperature one gram of a substance by one degree Celsius (J/g-°C). The relationship between quantity of heat (Q), specific heat (cp), mass (m) and temperature change (∆T) is mathematically expressed by the equation: assume that all the heat lost by the metal (Qx) is absorbed by the water and is equal to the heat gained by the water, (Qw). Since the specific heat of water is known the heat gained by water can be calculated: Q mc p T or Joules gJ / g CC The amount of heat needed to raise the temperature of 1 g of water by 1 degree Celsius is the basis of the calorie. Thus, the specific heat of water is exactly 1.00 cal/g∙ 0C. The SI unit of energy is the joule and it is related to the calorie by: 1 calorie = 4.184 J. Thus, the specific heat of water is also 4.184 J/g∙ 0C. The specific heat of a substance relates to its capacity to absorb heat energy. The higher the specific heat of a substance the more energy required to change its temperature. In this experiment, calorimetry is used to determine the specific heat of a metal. Heat energy is transferred from a hot metal to water until the metal and the water have reached the same temperature. This transfer is done in an insulated container to minimize heat losses to the surroundings. It is safe to Q w mw c p w Tw heat gained by water Q x m x c p x Tx heat lost by metal heat lost by metal is equal to the heat gained by water Qx Qw mx c p x Tx mw c p w Tw 𝑐𝑝𝑥 = 𝑚𝑤 𝑐𝑝𝑤 𝛥𝑇𝑤 − 𝑚𝑥 𝛥𝑇𝑥 This relationship can be used to calculate specific heat of a metal because both the mass of the metal and its temperature change can be measured.