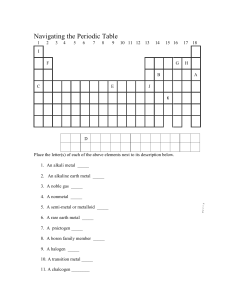
Homework H PHY3513 Due: 8 AM Nov. 24, 2009
advertisement
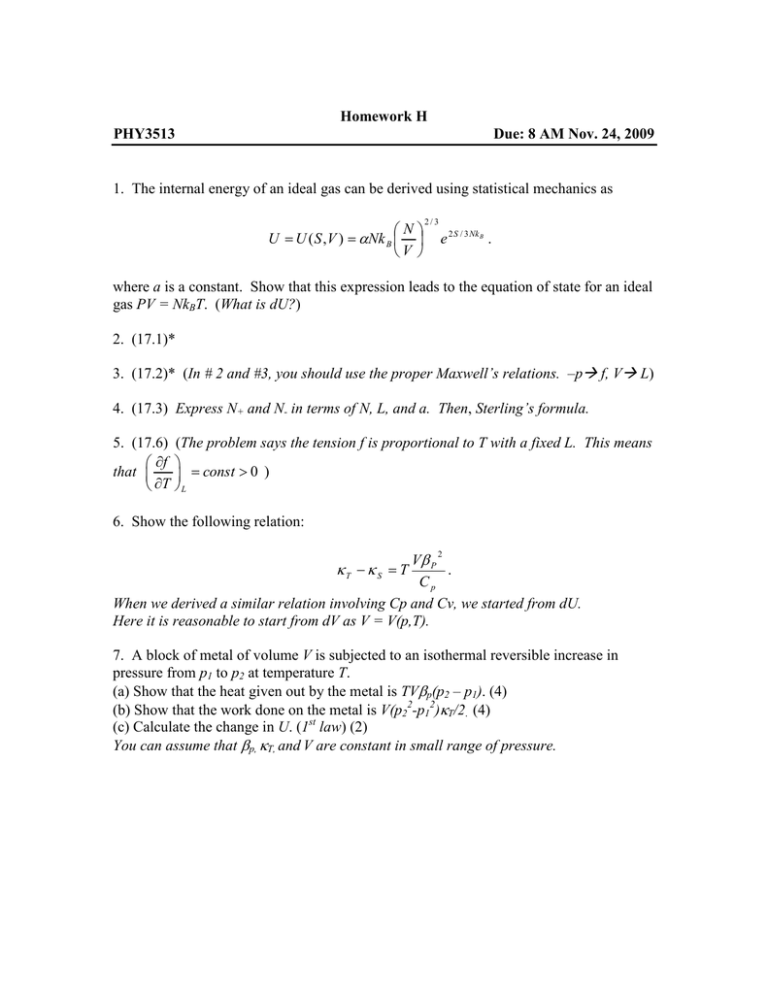
Homework H PHY3513 Due: 8 AM Nov. 24, 2009 1. The internal energy of an ideal gas can be derived using statistical mechanics as N U = U ( S , V ) = αNk B V 2/3 e 2 S / 3 Nk B . where a is a constant. Show that this expression leads to the equation of state for an ideal gas PV = NkBT. (What is dU?) 2. (17.1)* 3. (17.2)* (In # 2 and #3, you should use the proper Maxwell’s relations. –p f, V L) 4. (17.3) Express N+ and N- in terms of N, L, and a. Then, Sterling’s formula. 5. (17.6) ∂f that ∂T (The problem says the tension f is proportional to T with a fixed L. This means = const > 0 ) L 6. Show the following relation: Vβ P . Cp When we derived a similar relation involving Cp and Cv, we started from dU. Here it is reasonable to start from dV as V = V(p,T). κT − κ S = T 2 7. A block of metal of volume V is subjected to an isothermal reversible increase in pressure from p1 to p2 at temperature T. (a) Show that the heat given out by the metal is TVβp(p2 – p1). (4) (b) Show that the work done on the metal is V(p22-p12)κT/2. (4) (c) Calculate the change in U. (1st law) (2) You can assume that βp, κT, and V are constant in small range of pressure.