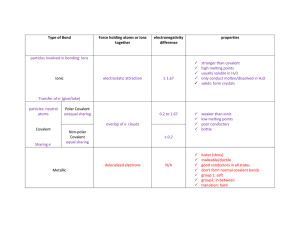
Chemical Periodicity Reuben Cauchi 1 Chemical periodicity Reuben Cauchi 2 Chemical periodicity Elements are laid out in: ➢ Strict order of Increasing atomic number. ➢ New row started when electrons start to enter a new main quantum shell. ➢ Elements whose atoms have a similar electronic configuration are placed in same vertical groups. A number of different ways of subdividing the table: ➢ Into vertical columns called groups (elements in a given group has a similar outer electronic configuration) ➢ Into horizontal rows called periods (elements in a given period have there outer electrons in the same principal energy level) ➢ Into s, p, d and f blocks (within a given block, the last electron added is within a sub-shell of that type) Outermost electron - the electron with the highest energy level Last electron - is the last electron to be placed when building up the electronic configuration of an element Reuben Cauchi 3 Chemical periodicity Reuben Cauchi 4 Chemical periodicity Reuben Cauchi 5 Periodicity of Physical Properties Atomic size: ➢ Decreases along a period ➢ Increase in proton number. ➢ Electrons enter same main quantum shell ➢ Therefore shielding remains approximately constant ➢ Thus force of attraction of nucleus on electrons increases ➢ Thus electrons are pulled nearer to the nucleus decreasing the atomic radius. ➢ Increases down a group ➢ Since electrons enter a new main quantum shell ➢ Outermost electrons arrange themselves in higher enegry levels ➢ Thus (although proton number (nuclear charge) increase), there is a smaller force of attraction on the outer electrons and hence atomic radius increase Reuben Cauchi 6 Periodicity of Physical Properties Calculated Atomic Radii (in Picometers) of the s-, p-, and d-Block Elements. The sizes of the circles illustrate the relative sizes of the atoms. Reuben Cauchi 7 Periodicity of Physical Properties 1st Ionisation energy: ➢ Increases along a period (In general, remember anomalies) ➢ Since electrons enter same main quantum shell ➢ Therefore shielding remains approximately constant ➢ But force of attraction of nucleus on electrons increases due to increase in proton number. ➢ Thus the outer electrons are pulled nearer to the nucleus requiring more energy to remove, i.e. 1st IE increases. ➢ Decreases down a group ➢ Since electrons enter a new main quantum shell ➢ Outermost electrons arrange themselves in higher enegry levels, increasing distance from the nucleus ➢ And thus, there is a smaller force of attraction on the outer electron, resulting in the outer electrons being further away from the nucleus and requiring less energy to remove , i.e. 1st IE decreases. Reuben Cauchi 8 Comparison of Melting points, Boiling points and conductivity Name of element Melting Point (°C) Boiling Point (°C) Electrical Conductivity Lithium 180.7 1342 Conductor Beryllium 1287 2970 Conductor Boron 2092 4002 Insulator Carbon 3552 4827 Conductor /Insulator Nitrogen -209.9 -195.7 Insulator Oxygen -218.2 -182.8 Insulator Fluorine -219.5 -188 Insulator Neon -248.5 -245.9 Insulator Sodium 98 883 Conductor Magnesium 649 1107 Conductor Aluminum 660.5 2467 Conductor Silicon 1410 2357 Semi-Conductor Phosphorus 44.3 280 Insulator Sulfur 119.2 444.8 Insulator Chlorine -100.3 -33.8 Insulator Argon -189.1 -185.6 Insulator Reuben Cauchi 9 Comparison of Melting points - Discussion Name of element Melting Point (°C) Lithium 180.7 Beryllium 1287 Boron 2092 Carbon 3552 Nitrogen -209.85 Oxygen -218.2 Fluorine -219.45 Neon -248.45 Sodium 98 Magnesium 649 Aluminium 660.5 Silicon 1410 Phosphorus 44.3 Sulfur 119.2 Chlorine -100.83 Argon -189.05 Reuben Cauchi 10 Comparison of Melting points - Discussion Name of element Melting Point (°C) Lithium 180.7 Beryllium 1287 Boron 2092 Carbon 3552 Nitrogen -209.85 Oxygen -218.2 Fluorine -219.45 Neon -248.45 Sodium 98 Magnesium 649 Aluminium 660.5 Silicon 1410 Phosphorus 44.3 Sulfur 119.2 Chlorine -100.83 Argon -189.05 Reuben Cauchi 11 Comparison of Melting points, Boiling points and conductivity Reuben Cauchi 12 Comparison of Melting points and Boiling points trends 6000 6 Temperature (°C) 5000 5 4000 4 3000 13 14 MP BP 2000 3 11 1000 12 15 7 8 9 10 0 0 5 10 15 16 17 18 20 -1000 Reuben Cauchi 13 Atomic Number From Li to Be: Inc. in mpt. and mpt. since: Comparison of Melting points and Boiling points trends effective nuclear charge and number of delocalised electrons increase. This results in an inc. in the overall force of attraction between the nucleus and the delocalised electrons, Hence more energy is needed to break the metallic bond and melt/boil. (Note that this has similar implication on hardness of metal, which increases across a period). B and C: Further increase in melting and boiling point since: Macromolecular structure i.e. melting/boiling requires breaking a large number of strong covalent bonds, hence higher then the previous metals B lower then C, since: B has only 3 covalent bonds, C has 4, and C has stronger attraction to bonding electrons since smaller, higher e.n.c. N to F and Ne: All are simple diatomic molecules (except Ne monoatomic) and thus have only induced dipole Van der Waal interactions, since they become smaller in size, the strength of the i.d. interactions decreases and hence less energy is needed to 14 Reuben Cauchi melt/boil. Comparison of Melting points and Boiling points trends From Na to Al: Inc. in mpt. and mpt. since: effective nuclear charge and number of delocalised electrons increase. This results in an inc. in the overall force of attraction between the nucleus and the delocalised electrons, Hence more energy is needed to break the metallic bond and melt/boil. (Note that this has similar implication on hardness of metal, which increases across a period). Si - Further increase since: Macromolecular structure i.e. melting/boiling requires breaking a large number of strong covalent bonds, hence higher then the previous metals P to Ar, Sudden drop since simple molecular(except Ar monoatomic) and thus have only induced dipole Van der Waal interactions, but: P is P4 while S is S8 hence: higher molar mass and larger in size, result in stronger induced dipole interactions in Sulfur and hence higher melting/boiling point. Cl2 and Ar, smaller in size, the strength of the i.d. interactions decreases and hence less energy is needed to melt/boil. Reuben Cauchi 15 Some noteworthy structures Shape of P4 Shape of S8 Silicon and carbon diamond have similar structures Reuben Cauchi 16 Electrical conductivity Metals: – All will conduct electricty because of the deloclaised electrons able to move freely through the structure. Non-metals: – Insulators, since no free charged particles able to move around the structure are present. Exceptions: – Graphite – conductor but only along plane, due to delocalised electrons present over and under the graphene sheets. Electrons cannot move easily between planes, since this would require moving the electrons from there orbitals. – Silicon – • Semiconductor – A semiconductor is a material whose conductivity falls between that of a conductor and an insulator. • In simple terms silicon can conduct electricity because its Si-Si bonds are weak enough such that some (very small numbers) of the bonds might “break” and hence its electrons able to move from one silicon to the other. Conductivity thus increases with temeprature (which is contrary to what is observed in typical conductors like metals) and can also be adjusted by introducing defects ex. Small amounts of impurities, which tend to increase conductivity and also lead to a number of possibilities like formation of diodes (LEDs) etc. Reuben Cauchi 17 Chemical trends: Reactions of elements with water Metals: – With water all react to form hydroxide and give off hydrogen, albeit under different conditions and at different rates. Grp 1 tend to easily react, quite exothermic such that hydrogen given off combust. Li(s) + H2O(l) → LiOH(aq)+ ½ H2 (g) Grp 2 slower, compounded further by somewhat impervious oxide layer, the more covalent the oxide the more impervious it tends to be and hence the more difficult the reaction. – Rate depends on activation enegry, down the group tends to decrease due to lower IE and weakening of metallic bond strength. – Freshly scratched Mg reacts slowly at RT but fast with steam (again formation of insoluble hydroxide layer tends to reduce rate of reaction) – Lower quite reactive at RT Mg(s) + 2H2O(l) → Mg(OH)2(s)+ H2 (g) – Be (and Al) tend not to react with wtaer at RT, but would react with steam at very high temperatures, giving the oxide (not hydroxide since this is unstable at these high temepratures) and hydrogen. Reuben Cauchi 18 Chemical trends: Reactions of elements with water Non-Metals: – B, N, O, Si, P, S no reaction in general due to either high Ea (ex. Triple bond or product not feasible). – C only at high T, C + H2O → CO + H2 – Fluorine and chlorine notice the differences in oxidation state: F2 + H2O → 2HF + ½ O2 Cl2 + H2O ⇌ HClO + HCl Why would fluorine and chlorine behave so differently (hint oxidative powers). Reuben Cauchi 19 Chemical trends: Reactions of elements with oxygen Metals: – All react directly with oxygen, giving normal oxide, while, in excess oxygen Na, Sr, Ba can give peroxide and K can give superoxide. (refer to s-block). Non-Metals: – All react directly except fluorine and chlorine. Oxides of these exist (ex. OF2 and Cl2O, Cl2O7) but being strong oxidising agents themselves, direct combination is not feasible. Reuben Cauchi 20 Trends – The oxides and hydroxides Period 3 M.Pt. (°C) Na2O 1132 MgO 2852 Al2O3 2040 SiO2 1700 P4O10 SO2 SO3 Cl2O 340 -72 17 – trimer 62.3 polymer -121 G Covalent State at R.T. S S S S S G L S Bonding ionic ionic Ionic Covalent Covalent Covalent Structure Giant ionic Giant ionic Ionic with degree of covalence Acidbase character Basic Basic Amphotheric Simple molecular MacroSimple Simple Large molecular molecular molecular molecule polymer Acidic Reuben Cauchi Acidic Acidic Acidic 21 Trends – The oxides and hydroxides Structure and bonding across period changes from ionic for metals to macromolecular for metalloids to simple molecular for non-metals. Structure of SiO2 Structure of P4O10 Structures of SO3 Reuben Cauchi 22 Trends – The oxides and hydroxides Some useful definitions: Acid → either donates an H+ or accepts a lone pair Base → either accepts H+ or donates a lone pair. Note trend: ionic = basic covalent = acidic, considerable degree of ionic/covalent character = amphoteric Metals: – Oxides of metals (Na and Mg) are ionic and basic in character due to the O2- anion. – With water: O2- + H2O → 2OHNa2O(s) + H2O(l) → 2NaOH(aq) MgO(s) + H2O(l) → Mg(OH)2(s) For Mg(OH)2 a white ppt. forms, since it is not that soluble – With acid: Magnesium oxide is less ionic Na2O + HCl → 2NaCl + H2O (higher polarising power), hence MgO + 2HCl → MgCl2 + H2O it is less basic Reuben Cauchi 23 Trends – The oxides and hydroxides Non-metals – Acidic oxides react with base to form a salt, (most non-metallic oxides are acidic no O2- ion present, some are neutral oxides ex. N2O) SiO2 will only react with hot conc. NaOH it won’t react with water nor is it soluble in it, due to macromolecular structure. Na2SiO3 –sodium silicate- could be soluble, depends on chain length. SiO2(l) + NaOH(l) → Na2SiO3 + H2O(l) Is this a redox reaction? P4O10 (phosphorus(V) oxide) Phosphoric(V) acid With water: P4O10(s) + 6H2O(l) → 4 H3PO4(aq) With base: P4O10(s) + 12 NaOH (aq) → 4 Na3PO4(aq) + 6H2O(l) Sodium phosphate(V) Cl2O (Chlorine(I) oxide) With water: Cl2O(g) + H2O(l) → 2HClO(aq) With base: Cl2O(g) + 2NaOH(aq) → 2NaClO(aq) + H2O(l) Chloric(I) acid is unstable in the presence of Cl–: Chloric(I) acid Sodium chlorate(I) HClO + C– + H+ ⇌ Cl2 + H2O Reuben Cauchi 24 Trends – The oxides and hydroxides SO2 (Sulfur(IV) oxide) Note the difference in acid strength of the two sulfur oxides With water: SO2(g) + H2O(l) ⇌ H2SO3(aq) With base: SO2(g) + 2 NaOH(aq) → Na2SO3(aq) + H2O(l) Sodium sulfate(IV) Sulfuric(IV) acid SO3 (Sulfur(VI) oxide) With water: SO3(l) + H2O(l) → H2SO4(aq) Sulfuric(VI) acid With base: SO3(l) + 2 NaOH(aq) → Na2SO4(aq) + H2O(l) Sodium sulfate(VI) Amphoteric oxides: with water: Al2O3 Tend to have intermediate bonding, between ionic and covalent, hence the “intermediate” acid-base behaviour No simple reaction, does not dissolve in water – hint towards less ionic character With acid: Al2O3(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2O(l) Sodium tetrahydroxoaluminate With base: Amphoteric (a species which can both act as an acid and as a base) Al2O3(s) + 2 NaOH(aq) + H2O(l) → 2Na[Al(O H)4](aq) Note that the Al3+ is acting as a bae by accepting a lone pair from OH– Reuben Cauchi 25 Trends – The hydroxides All the hydroxides are ionic, hence solid at room temeprature Degree of ionic character decreases from NaOH to Al(OH)3. Hence become less basic, eventually amphoteric (Al(OH)3. NaOH with water simply dissociates into Na+ and OH-, so soluble it is a deliquescent substance i.e. absorb moisture from air and dissolves in it. Mg(OH)2 and Al(OH)3 are thermally unstable (decompose to oxide) and are insoluble, although Al(OH)3, being amphoteric dissolves in xs. OH-. Basic hydroxides: NaOH NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l) Mg(OH)2 Mg(OH)2(s) + 2HCl(aq) → MgCl2(aq) + H2O(l) Amphoteric hydroxide - Al(OH)3 : With acid: Al(OH)3 (s) + 3HCl(aq) → AlCl3(aq) + 3H2O(l) With base: Al(OH)3 (s) + NaOH(aq) → Na[Al(O H)4](aq) Amphoteric (a species which can both act as an acid and as a base) Reuben Cauchi 26 Chemical trends – The Chlorides Period 3 NaCl MgCl2 AlCl3 SiCl4 PCl3 PCl5 M.Pt. (°C) 808 714 178* -70 -92 160* Simple molecular Solid - ionic (PCl4+ PCl6-) Gas – covalent simple molecular (PCl5) Molecular with some ionic character Giant ionic Giant ionic Bonding ionic Ionic with a degree of covalence Mostly Covalent Covalent Covalent Covalent, ionic between particles in solid State at R.T. S S S L L S Reactions with water Na+ and Cl- Mg2+ and Cl- Al(OH)3 and HCl Hydrated SiO2 and HCl H3PO3 and HCl POCl3 and HCl Structure Simple molecular *Sublimes, if hydrated it decomposes to oxide and HCl on heating. Cauchi when molten, not AlCl 3, NaCl and MgCl2, being ionic will conductReuben electricty 27 Trends – The Chlorides – All elements react directly with chlorine (direct combination), sometimes explosively. – Chlorine, being a powerful oxidising agent will usually act as an oxidising agent, itself reduced to the -1 oxidation state. – Except when reacting with fluorine, where it will form species with positive oxidation state, sicne fluorine is more electronegative. – We shall observe a simialr trend as to that in oxides, from ionic to covalent with inetrmediate bonding. – However, the chloride is more polarisable and hence transition is more sudden and before. – Hence AlCl3 can be considered as mainly covalent, especially when considering its structure. Na to Al, cation becomes more polarising (higher charge density), this distorts the electron cloud of the chloride anion more, increaseing degree of covalent character. Reuben Cauchi 28 Trends – The Chlorides Melting points trend: Na to Al chloride becomes more covalent thus the attraction between non-neighbouring oppositely charged ions decreases, decreaseing energy needed to break attractions hence lowering melting points. SiCl4, PCl3 simple molecular, SiCl4 has string id VdW interactions, hence stronger attractions overall (even though PCl3 is polar), thus higher melting point, PCl5, high melting point can’t be explained only due to increase in chlorines: PCl5 solid at RT since it becomes ionic in the solid state forming [PCl4]+[PCL6]- ions In the vapour phase it will form the PCl5 molecule (if molten or dissolved in a solvent it does not react with it will conduct electricity) Reuben Cauchi 29 Trends – The Chlorides AlCl3 In the solid state each Al atom is 6-coordinated with Cl atoms (each Al atom is surrounded by 6 Cl atoms) forming a sheet-like layer On melting/subliming at low temperatures, the dimer Al2Cl6 forms: ⇌ 2 Increasing temperature shifts equilbirum forward, what does this say about the enthalpy of reaction? What is it so? What happens on increaseing pressure? Reuben Cauchi 30 Trends – The Chlorides Trends with water: Purely ionic chlorides will simply dissociate in water forming ions, resulting in neutral solutions: NaCl(s) + aq → Na+(aq) + Cl– (aq) MgCl2 and AlCl3 will first dissociate into salts but then undergo salt hydrolysis, due to the higher charge density of the cations: MgCl2(s) + aq → Mg2+(aq) + 2Cl–(aq) AlCl3(s) + aq → Al3+(aq) + 3Cl–(aq) But then will undergo salt hydrolysis to varying extents, mainly depending on charge denisty of cation, resulting in somewhat acidic solutions: [Mg(H2O)6]2+(aq) + H2O(l) ⇌ [Mg(H2O)5(OH)] +(aq) + H3O+(aq) [Al(H2O)6]3+(aq) + 3H2O(l) ⇌ [Al(H2O)5(OH)3] (s) + 3H3O+(aq) The more polarising cation of Al3+, attracts the electron cloud of water more towards it, hence dissociating more easily the O-H bond releasing more H+ ions, Resulting in more acidic solutions (pH 2-3, depending on T and concentration) Salt hydrolysis - in the case of Mg, Mg is not that polarising and so this equilbirum lies mainly to the left, It still occurs such that MgCl2 solutions are (very slightly) acidic (pH around 6, depending on T and concentration) Reuben Cauchi 31 Trends – The Chlorides Trends with water: Purely ionic chlorides will simply dissociate in water forming ions, resulting in neutral solutions: NaCl(s) + aq → Na+(aq) + Cl– (aq) MgCl2 and AlCl3 will first dissociate into salts but then undergo salt hydrolysis, due to the higher charge density of the cations: MgCl2(s) + aq → Mg2+(aq) + 2Cl–(aq) AlCl3(s) + aq → Al3+(aq) + 3Cl–(aq) But then will undergo salt hydrolysis to varying extents, mainly depending on charge denisty of cation, resulting in somewhat acidic solutions: [Mg(H2O)6]2+(aq) + H2O(l) ⇌ [Mg(H2O)5(OH)] +(aq) + H3O+(aq) [Al(H2O)6]3+(aq) + 3H2O(l) ⇌ [Al(H2O)5(OH)3] (s) + 3H3O+(aq) The more polarising cation of Al3+, attracts the electron cloud of water more towards it, hence dissociating more easily the O-H bond releasing more H+ ions, Resulting in more acidic solutions (pH 2-3, depending on T and concentration) Salt hydrolysis - in the case of Mg, Mg is not that polarising and so this equilbirum lies mainly to the left, It still occurs such that MgCl2 solutions are (very slightly) acidic (pH around 6, depending on T and concentration) With AlCl3 if water is dropped onto the solid, HCl gas would be given off, but not if dissolved in xs. Reuben Cauchi water 32 Trends – The Chlorides Trends with water: Purely covalent chlorides will react with water to give HCl: SiCl4(g) + 2H2O(l) → SiO2(s) + 4HCl (g) If the water is limiting fumes of HCl are given off, SiCl4 fumes in air, due tio reacting with moisture. If xs. Water, then the HCl would dissolve and white fumes might not be observed. The reaction really is a step-wise nucleophilic substitution: SiCl4(g) + H2O(l) → SiCl3(OH) + HCl (g) Hydroxide unstable decomposes to oxide Followed by the decomposition of the unstable hydroxide to the silicon oxide: Si(OH)4 → SiO2(s) + 4H+ Note that CCl4 will not undergo this reaction due to steric hinderance, since the C atom is smaller and thus the 4 Cl atoms will “obstruct” (sterically hinder) the water from attacking the nucelophile (and also the high activation energy needed to accept a lone pair from water in the next available empty orbital, the 3s). Reuben Cauchi 33 Trends – The Chlorides Trends with water: Simialr to SiCl4, both phosphorus chlorides react violently with water giving off white fumes of HCl, which you can test with damp blue litmus, PCl3: PCl3(g) + 3H2O(l) → H3PO3 (s) + HCl (g) If water is in xs. Just change the state symbols: PCl3(g) + 3H2O(l) → H3PO3 (aq) + HCl (aq) PCl5: PCl5(s) + 4H2O(l) → POCl3 (s) + 2HCl (g) If water is in excess and the solution is heated the POCl3 would also react: POCl3 (s) + 3H2O(l) → H3PO4 (aq) + 3HCl(aq) Overall reaction in xs. water and heating: PCl5 (s) + 4H2O(l) → H3PO4(aq) + 5HCl(aq) Reuben Cauchi 34 Acidity of trivalent metal ions (Fe3+, Al3+, Cr3+): The Effect of the Charge and Radius of a Metal Ion on the Acidity of a Coordinated Water Molecule. The contours show the electron density on the O atoms and the H atoms in both a free water molecule (left) and water molecules coordinated to Na+, Mg2+, and Al3+ ions. These contour maps demonstrate that the smallest, most highly charged metal ion causes the greatest decrease in electron density of the O–H bonds of the water molecule. Due to this effect, the acidity of hydrated metal ions increases as the charge on the metal ion increases and its radius decreases. The positive charge on the aluminium ion attracts electron density from the oxygen atoms, which shifts electron density away from the O–H bonds. The decrease in electron density weakens the O–H bonds in the water molecules and makes it easier for them to lose a proton. https://chem.libretexts.org/Courses/Mount_Royal_University/Chem_1202/Unit_2%3A_Acids_and_Bases/15.07%3A_Ions_as_Acids_and_Bases Reuben Cauchi Note on salt hydrolysis Acidity of metal ions with high charge density, namely trivalent cation (Al3+, Fe3+, Cr3+): https://chem.libretexts.org/Courses/Mount_Roya l_University/Chem_1202/Unit_2%3A_Acids_and_B ases/15.07%3A_Ions_as_Acids_and_Bases The high charge density of the trivalent cation pulls electron density of the water ligands towards it resulting in weakening of the O-H bond. Thus the H-atoms in the water can more easily be lost (i.e. become acidic), such that water molecules act as base, donating a lone pair and extracting an H+ from the complex. [Al(H2O)6]3+(aq) + H2O(l) ⇌ [Al(H2O)5(OH)]2+(aq) + H3O+(aq) The H3O+ generated results in lowering of the pH of the solution. Two further equilibria are possible with a lower Ka, as discussed for acids with multiple basicity. Adding a less acidic (more basic) substance would potentially shift these equilibria forward: [Al(H2O)5(OH)]2+(aq) + H2O(l) ⇌ [Al(H2O)4(OH)2]+(aq) + H3O+(aq) [Al(H2O)4(OH)2]+(aq) + H2O(l) ⇌ [Al(H2O)3(OH)3](s) + H3O+(aq) Reuben Cauchi Chemical trends – The Hydrides Period 3 NaH MgH2 SiH4 PH3 H2S HCl M.Pt. (°C) 800* 327* -185 -133 -82 -114 Structure Giant ionic Giant Ionic Simple molecular Simple molecular Simple molecular Simple molecular Bonding Ionic Mainly Ionic Covalent Covalent Covalent Covalent State at R.T. S S G G G G Reactions with water NaOH and H2 Mg(OH)2 and H2 Hydrated SiO2 and H2 - Low conc. Of H3O+, S2and HS- H3O+, and Cl- *Decompose before boiling Reuben Cauchi 37 Chemical trends – The Hydrides Structures: SiH4 – sialne – non polar induced dipole VdW interactions only PH3 – phosphine – polar induced and permanent dipole VdW interactions and slightly larger mass Reuben Cauchi H2S –hydrogen sulfide – polar induced and permanent dipole VdW interactions and slightly larger mass and more electronegative S = larger dipole 38 Chemical trends – The Hydrides Ionic hydrides (NaH and MgH2) will react with water to form basic solution in what is both an acid base and a redox reaction: H– + H2O(l) → H2(g) + OH– SiH4, although not ionic reaction is simialr, due to nucleophilic attack by the water, which form Si(OH)4, but then decomposes to SiO2, simialr to SiCl4: SiH4(g) + H2O(l) → H2(g) + SiO2(s) PH3: No reaction unless with high pressure and temeprature H2S: H2S(g) + H2O(l) ⇌ HS–(aq) + H3O+(aq) HS–(aq) + H2O(l) ⇌ S2–(aq) + H3O+(aq) HCl: HCl(g) + H2O(l) → Cl–(aq) + H3O+(aq) Reuben Cauchi 39