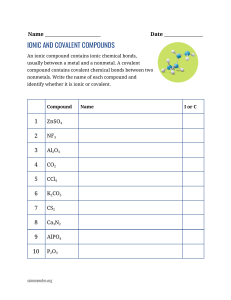
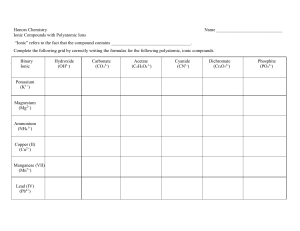
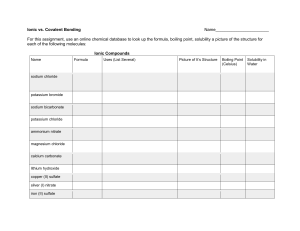
Quiz – Ionic & Covalent Bonds Name ______________________ Write out the name for the following: 1. N2O (laughing gas) ______________________________________ 2. AS2O3 _____________________________________ 3. Na2CO3 ____________________________________ 4. FeSO4 ______________________________________ 5. CuF2 _______________________________________ Write out the formula for the following: 6. Sulfurous acid _______________________________ 7. Carbon tetrachloride _______________________________ 8. Magnesium nitride ________________________________ 9. Mercury (I) sulfide _________________________________ 10. Copper (II) nitrate _________________________________ Draw out the dot structure for the following: 11. CCl4 12. C2H4 Identify the following as Ionic or Covalent 13. Calcium Carbonate ________________________ 14. Iron (III) oxide ____________________________ 15. Iodine pentaflouride ______________________ 16. NF4 ___________________________________ 17. KCl ___________________________________ Identify the type of bond between the following pairs of elements. (ionic, polar, nonpolar) 18. Na – Cl __________________________________________ 19. N – O ___________________________________________ 20. H – H ____________________________________________ 21. What is the formula for nitric acid? _________________________________ 22. Write out the structural formula for ammonia (NH3). Show all dots or dashes to represent bonds.