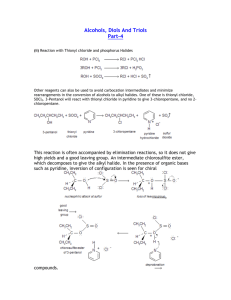
FUNCTIONAL GROUPS ORGANIC HALIDES Properties • Halogen makes the molecule more polar, increasing strength of the intermolecular forces – Higher boiling points than their corresponding hydrocarbons – More soluble in polar solvents • Molecules with more halogens are more polar Preparing Organic Halides Recall: Substitution reaction produce halides in alkanes and aromatic hydrocarbons Preparing Organic Halides Recall: alkyl halides are produced in halogenation reactions (addition) with alkenes/alkynes Elimination Reactions • Preparing alkenes from alkyl halides • Most common method of preparing alkenes • Alkyl halides can eliminate a hydrogen and a halide ion from adjacent carbon atoms forming a double bond. • Presence of a hydroxide ion is required ALCOHOLS • Organic compounds containing a hydroxyl group -OH • E.g. ethanol, cholesterol, retinol (vitamin A) Naming: • -ol suffix e.g. methane + OH = methanol Polyalcohols • Alcohols containing more than one -OH group • -diol, -triol suffix • Or hydroxy prefix 1,2-dihydroxyethane 1,2,3-trihydroxypropane Properties of Alcohols • More polar and can hydrogen bond • Higher boiling points • More soluble in polar solvents • Long-chain alcohols are nonpolar (hydrocarbon portion) and polar (-OH) • Ideal solvents in organic reactions because they will dissolve both polar and nonpolar compounds REACTIONS INVOLVING ALCOHOLS Hydration Reactions (Addition) (Formation of Alcohol) • Alkene + water --> alcohol • Follows Markovnikov’s rule Combustion of Alcohols Elimination Reactions Dehydration (Condensation) Reactions: • Under certain conditions alcohols can decompose to produce alkenes and water • A catalyst (sulfuric acid) removes a hydrogen atom and a hydroxyl group from neighbouring carbons • Resulting in C=C and H2O ETHERS • Molecules with C-O-C group • More polar than hydrocarbons • But, unlike alcohols, ethers cannot hydrogen bond Naming: • Add oxy to the prefix of the smaller hydrocarbon group and join it to the alkane name of the larger hydrocarbon group • E.g. CH3-O-C2H5 is methoxyethane Condensation Reactions (Formation of Ethers) • When two alcohols combine, an ether and water are formed ALDEHYDES AND KETONES Ketone: Molecule with a carbonyl group (C=O) between two carbon atoms. Alkane name with -one suffix Aldehyde: Molecule with a carbonyl group (C=O) on a terminal carbon. Alkane name with -al suffix Properties of Aldehydes and Ketones • Polar but no Hydrogen Bonding • Lower boiling point and less soluble in water than alcohols (no -OH) • More polar than hydrocarbons (higher boiling points and more soluble) • Good solvents (both polar and nonpolar) Oxidation Reactions • Alcohol + an oxidizing agent (removes electrons) to form an aldehyde or ketone and water • The oxidizing agent removes two H-atoms (one from the -OH group and one from the adjacent carbon) resulting in C=O and H2O Oxidizing agent No available H-atom Hydrogenation Reactions • The C=O double bond can undergo an addition reaction with hydrogen to form an -OH group. • Aldehydes always produce 1˚ alcohols • Ketones always produce 2˚ alcohols CARBOXYLIC ACIDS • Molecules with a carboxyl group - COOH • E.g. lactic acid, citric acid • NAMING: • Alkane name with -oic acid • E.g. methanoic acid Properties of Carboxylic Acids • Polar • Can hydrogen bond • Similar properties to alcohols (smaller members are soluble in water, larger members are insoluble) • pH < 7 (H-atom in -OH group) Oxidation Reactions Recall: A 1˚ alcohol can be oxidized to form an aldehyde. An aldehyde can be further oxidized to form a carboxylic acid A ketone cannot be oxidized because there is no free H-atom Oxidation Reaction ESTERS • NAMING: • 1st name is the part single bonded to only 1 Oxygen (the part that was originally the alcohol) it gets named like a side chain with the ending ‘-yl’ • 2nd name is the part double bonded to the Oxygen it is named with the ending ‘-oate’ EXAMPLES Esterfication (Formation of Esters) Recall: acid + base --> salt + water (neutralization reaction) Carboxylic acid + alcohol --> ester + water Properties of Esters • Similar to carboxylic acids, but lacking -OH group • Esters are less polar (less soluble in water), lower boiling points (no H-bonds) • Not acidic •Many have characteristic scents AMINES • An ammonia molecule in which one or more H-atoms are substituted by alkyl or aromatic groups Naming: • Amino + alkane name OR • Alkyl group + amine Naming 2˚ and 3˚ Amines • N- prefix used for the substituted groups on the nitrogen atom • Alkyl groups are listed alphabetically N,N-dimethyl-2-aminopropane Properties of Amines • N-C, and N-H polar bonds • H-bonding occurs but N-H is less polar than O-H Synthesizing Amines from Alkyl Halides • Alkyl halide + ammonia --> 1˚ amine + HX • Alkyl halide + 1˚ amine --> 2˚ amine + HX • Alkyl halide + 2˚ amine --> 3˚ amine + HX Synthesizing Amines from Alkyl Halides AMIDES • Similar to esters, except N instead of O Recall: Carboxylic acids + alcohols --> esters + water Carboxylic acids + ammonia/1˚/2˚ amines--> amides + water Naming Amides • 1st part of the name is from the amine • 2nd part is the ending of the acid name changed from -oic to -amide • Alphabetical order with N- groups N-ethyl propanamide Summary