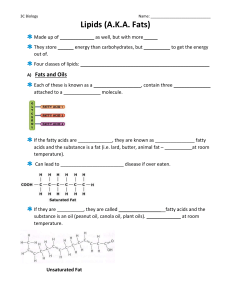
Fats and Oils Fats and Oils are essential components of our diet, serving as concentrated sources of energy and carriers for fat-soluble vitamins. Chemically, they belong to the lipid family and are composed of triglycerides. The primary distinction between fats and oils lies in their physical state at room temperature; fats are typically solid, while oils are liquid. This difference is attributed to the saturation levels of fatty acids within the molecules. Fats tend to have saturated fatty acids, which pack closely and solidify, while oils contain unsaturated fatty acids, maintaining a more liquid form due to their structure and reduced intermolecular forces. Fatty Acids are organic molecules that serve as building blocks of fats and lipids. Chemically, they consist of a hydrocarbon chain with a carboxyl group (-COOH) at one end. The hydrocarbon chain can vary from 4-36 carbons long and may be saturated or unsaturated. Saturation: Fatty acids can be classified into saturated and unsaturated types. Saturated fatty acids contain only single bonds between carbon atoms, leading to a straight molecular structure that allows them to pack tightly, resulting in a solid state at room temperature. In contrast, unsaturated fatty acids have one or more double bonds, preventing close packing and maintaining a liquid state. Unsaturated fats are mostly found in vegetable oils (liquid) while saturated fats mainly comes from animal fats (solid) Glycerol is a trihydroxy sugar alcohol with the chemical formula C3H8O3. It is a colorless, odorless, and sweet-tasting viscous liquid. The chemical structure of glycerol consists of three hydroxyl (OH) groups attached to a propane backbone.Its systematic name is 1,2,3-propanetriol. Triglycerides are esters composed of three fatty acids and a glycerol molecule. The chemical reaction involved in the formation of a triglyceride is known as esterification, resulting in the formation of the triglyceride molecule along with the release of three water molecules. Triglycerides are hydrophobic, meaning that they are unable to dissolve. This is due to the nonpolar nature of the hydrocarbon chains in the fatty acids. Chemical reaction of Coconut Oil