Materials Science Exercises: Crystal Structure, Density, Stress
advertisement

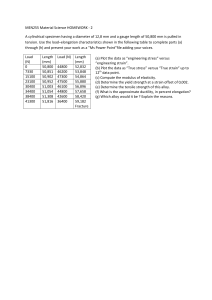
EXERCISES 1. Iron exhibits bcc structure at room temperature. Above 900oC, it transforms to fcc structure. Calculate the ratio of density of iron at room temperature to that at 900oC (assuming molar mass and atomic radius of iron remains constant with temperature). 2. Niobium crystallizes in body centred cubic structure. If density is 8.55 g/cm3, calculate atomic radius (Ao) of niobium using its atomic mass 92.90 (AN = 6.02×1023). 3. Calculate the radius of an iridium atom, given that Ir has an FCC crystal structure, a density of 22.4 g/cm3, and an atomic weight of 192.2 g/mol (AN = 6.02×1023) 4. Calculate the radius of a vanadium atom, given that V has a BCC crystal structure, a density of 5.96 g/cm3, and an atomic weight of 50.9 g/mol. (AN = 6.02×1023). 5. Iron has a BCC crystal structure, an atomic radius of 0.124nm, and an atomic weight of 55.85 g/mol. Compute and compare its theorical density with the experimental value found inside the front cover (AN = 6.02×1023). 6. An element crystallizes in a structure having FCC unit cell with a lattice constant of 200pm. If 200g of this element contains 24×1023 atoms, calculate the density (in g/cc) of the elements. (AN = 6.02×1023). 7. A metal has a body centered cubic (bcc) strtucture with a lattice constant of 288pm. The density of the metal is 7.2g/cm3. How many atoms present in 500g of the metal? 8. At room temperature, sodium crystallizes in body centred cubic lattice. Calculate theoretical density (g/cm3) of sodium (Na=23, rNa=1.02Ao). (AN = 6.02×1023). 9. A specimen of aluminium having a rectangular cross section 10mm×12.7mm and a Young’s modulus of 69 GPa is pulled in tension with 35,500N force, producing only elastic deformation. Calculate the resulting strain. 10. A cylindrical specimen of a titanium alloy having an elastic modulus of 197GPa and an original diameter of 3.8mm will experience only elastic deformation when a tensile load of 2000N is applied. Compute the maximun length of the specimen before deformation if the maximum allowable elongation is 0.42mm. 11. Consider a cylindrical specimen of some hypothetical metal alloy that has a diameter of 8.0mm. A tensile force of 1000N produces an elastic reduction in diameter of 2.8×10-4mm. Compute the modulus of elasticity for this alloy, given that Poisson’s ratio is 0.30. 12. A brass alloy is known to have a yield strength of 275MPa, a tensile strength of 380 MPa, and an elastic modulus of 103 GPa. A cylindrical specimen of this alloy 12.7mm in diameter and 250mm long is stressed in tension and found to elongate 7.6mm. On the basis of the information given, it is possible to compute the magnitude of the load that is necessary to produce this change in length? If so, calculate the load. If not, explain why. 13. (a) A force of 100,000N is applied to an iron bar with a cross-sectional area of 10mm×20mm and having a yield strength of 400MPa and a tensile strength of 480MPa. Determine whether the bar will plastically deform and whether the bar will experience necking. (b) Calculate the maximum force that a 0.2-in. diameter rod of Al2O3, having a yield strength of 35, 000 psi, can withstand with no plastic deformation. Express your answer in pounds and Newtons. 14. A cylindrical specimen of alumium having a diameter of 0.505 in. (12.8mm) and a gauge length of 2.000 in. (50.800mm) is pulled in tension. Use the load elongation characteristics shown in the following table to complete parts (a) and through (e) (a) Plot the data as engineering stress versus engineering strain (b) Compute the modulus of elasticity (c) Determine the yield strength at a strain offset of 0.002 (d) Determine the tensile strength of this alloy (e) What is the approximate ductility, in percent elongation? Load Length N lbf mm in. 0 0 50.800 2.000 7,330 1,650 50.851 2.002 15,100 3,400 50.902 2.004 23,100 5,200 50.952 2.006 30,400 6,850 51.003 2.008 34,400 7,750 51.054 2.010 38,400 8,650 51.308 2.020 41,300 9,300 51.816 2.040 44,800 10,100 52.832 2.080 46,200 10,400 53.848 2.120 47,300 10,650 54.864 2.160 47,500 10,700 55.880 2.200 46,100 10,400 56.896 2.240 44,800 10,100 57.658 2.270 42,600 9,600 58.420 2.300 36,400 8,200 59.182 2.330 Fracture 15. For a bronze alloy, the stress at which plastic deformation begins is 280MPa and the modulus of elasticity is 115 GPa. (a) What is the maximum load that may be applied to a specimen with a cross-sectional arera of 325mm2 without plastic deformation? (b) If the original specimen length is 120mm, what is the maximum length to which it may be stretched without causing plastic deformation? 16. Consider a cylindrical specimen of a steel alloy (Figure 6.21) 15.0mm in diatmeter and 75mm long that is pulled in tension. Determine its elongation when a load of 20,000N is applied. 17. A cylindrical specimen of a hypothetical metal alloy is stressed in compression. If its original and final diameters are 20.000 and 20.025mm, respectively, and its final length is 74.96mm, compute its original length if the deformation is totally elastic. The elastic and shear moduli for this alloy are 105GPa and 39.7 GPa, respectively. 18. A pipe has an outside diameter of 20mm, an inside diameter of 10mm and length 0.30m and it supports a compressive load of 50kN. The pipe shortens by 0.6mm when the load is applied. Determine: (a) The compressive stress (b) The compressive strain in the pipe when supporting this load 19. When a circular hole of diameter 40mm is punched out of a 1.5mm thick metal plate, the shear stress needed to cause fracture is 100MPa. Determine: (a) The minimum force to be applied to the punch (b) The compressive stress in the punch at this value 20. A force of 25kN applied to a piece of steel produces an extension of 2mm. Assuming the elastic limit is not exceeded, determine: (a) The force required to produce an extension of 3.5mm (b) The extension when the applied force is 15kN. 21. A bar of thickness 20mm and having a rectangular cross-section carries a load of 82.5kN. Determine: (a) The minimum width of the bar to limit the maximum stress to 150MPa (b) The modulus of elasticity of the material of the bar if the 150mm long bar extends by 0.8mm when carrying a load of 200kN 22. A current density of 0.05 A/cm2 is applied to a 150cm2 cathode. What period of time (s) is required to plate out a 1-mm-thick coating of silver onto the cathode? (dAg = 10.5g/cm3, MAg = 108g/mol, F = 96500C/mol) 23. A 1-m-square steel plate is coated on both sides with a 0.005-cm-thick layer of zinc. A current density of 0.02A/cm2 is applied to the plate in an aqueous solution. Assuming that the zinc corrode uniformly, determine the length of time required before the steel is exposed. 24. A corrosion cell is composed of a 300cm2 copper sheet and a 20cm2 iron sheet, with a current density 0f 0.6A/cm2 applied to the copper. Which material is the anode? Why? What is the rate of loss of metal from the anode per hour? 25. At room temperature the electrical conductivity and the electron mobility for 2 copper are 6 × 107 (Ωm)−1 and 0.0030 𝑚 ⁄𝑉𝑠 respectively. (a) Compute the number of free electrons per cubic meter for copoper at room temperature. (b) What is the number of free electrons per copper atom? Assume copper has a density of 8.9 g/cm3, q = 1.6 × 10−19 𝐶, AN = 6.02×1023 26. A cylindrical metal wire 2mm (0.08in.) in diameter is required to carry a current of 10A with a minimum of 0.03V drop per foot (300mm) of wire. Which of the metals and alloys listed in the table below are possible candidates? 27. Metal Electrical Conductivity [(𝛀𝐦)−𝟏 ] Silver 6.8 × 107 Copper 6.0 × 107 Gold 4.3 × 107 Aluminium 3.8 × 107 Brass (70Cu-30Zn) 1.6 × 107 Iron 1.0 × 107 Platinium 0.94 × 107 Plain carbon steel 0.6 × 107 Stainless steel 0.2 × 107 A 1-m-square steel plate is coated on both sides with a 0.005-cm-thick layer of zinc. A current density of 0.02 A/cm2 is applied to the plate in an aquaeous solution. Assuming that the zinc corrodes uniformly, determine the length of time required before the steel is exposed. (d Zn = 7.14g/cm3) 28. The metal nickel crystallizes in a face centred cubic structure. Its density is 8.9 g/cm3. Calculate: a. The lattice constant (nm) b. The radius of the nickel atom (Ao) Given that the atomic weight of Ni is 58.89, (AN = 6.02×1023). 29. Magnesium is Hcp with c/a=1.624, density = 1.74g/cm3. Find the atomic radius (nm) of magnesium. Mg = 24, (AN = 6.02×1023). 30. A tube of outside diameter 60mm and inside diameter 40mm is subjected to a tensile load of 60 kN. Determine the stress in the tube.