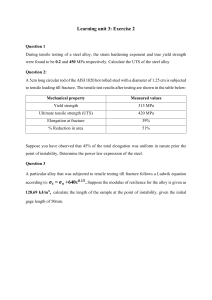
MECHANICAL PROPERTIES 1. A specimen of copper having a rectangular cross section 15.2 mm × 19.1 mm is pulled in tension with 44,500 N force, producing only elastic deformation. Calculate the resulting strain. 2. A cylindrical specimen of a nickel alloy having an elastic modulus of 207 GPa and an original diameter of 10.2 mm experiences only elastic deformation when a tensile load of 8900 N is applied. Compute the maximum length of the specimen before deformation if the maximum allowable elongation is 0.25 mm . 3. A cylindrical specimen of a brass alloy having a length of 100 mm must elongate only 5 mm when a tensile load of 100,000 is applied. Under these circumstances, what must be the radius of the specimen? 4. A tensile test is performed on a metal specimen, and it is found that a true plastic strain of 0.16 is produced when a true stress of 500 MPa is applied; for the same metal, the value of K in the quation is 825 MPa). Calculate the true strain that results from the application of a true stress of 600 MPa . 5. A 10-mm-diameter Brinell hardness indenter produced an indentation 2.50 mm in diameter in a steel alloy when a load of 1000 kg was used. Compute the HB of this material. What will be the diameter of an indentation to yield a hardness of 300 HB when 500kg load is used ? DISLOCATIONS AND STRENGTHENING MECHANISMS 6 a) Calculate the fraction of atom sites that are vacant for copper (Cu) at its melting temperature of 1085°C (1358 K). Assume an energy for vacancy formation of 0.90 eV/atom. (b) Repeat this calculation at room temperature (298 K). (c) What is ratio of N𝜐/N(1358 K) and N𝜐/N (298 K) ? 7 Some hypothetical alloy is composed of 25 wt% of metal A and 75 wt% of metal B. If the densities of metals A and B are 6.17 and 8.00 g/cm3, respectively, and their respective atomic weights are 171.3 and 162.0 g/mol, determine whether the crystal structure for this alloy is simple cubic, face centered cubic, or bodycentered cubic. Assume a unit cell edge length of 0.332 nm. 8 Niobium forms a substitutional solid solution with vanadium. Compute the number of niobium atoms per cubic centimeter for a niobium vanadium alloy that contains 24 wt% Nb and 76 wt% V. The densities of pure niobium and vanadium are 8.57 and 6.10 g/cm3, respectively. 9 Consider a BCC iron–carbon alloy that contains 0.2 wt% C, in which all the carbon atoms reside in tetrahedral interstitial sites. Compute the fraction of these sites that are occupied by carbon atoms. 10 Calculate the unit cell edge length for an 80 wt% , Ag–20 wt% Pd alloy. All of the palladium is in solid solution, the crystal structure for this alloy is FCC, and the room-temperature density of Pd is 12.02 g/cm3.