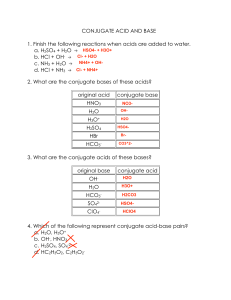
CHM202 Unit 3 Practice Test 1. Which one of the following is not a strong acid? a. b. c. nitric acid, HNO3 sulfuric acid, H2SO4 carbonic acid, H2CO3 d. e. hydrochloric acid, HCl perchloric acid, HClO4 2. Which of the following is the conjugate base of the hydrogen carbonate ion, HCO 3−? a. b. c. H2CO3 HCO3− CO32− d. e. OH− H3CO3+ 3. Use the following acid ionization constants to identify the correct decreasing order of base strengths for the conjugate bases. HF HNO2 HCN a. b. c. Ka = 7.2 10−4 Ka = 4.5 10−4 Ka = 6.2 10−10 CN− NO2− F− NO2− F− CN− F− CN− NO2− d. e. F− NO2− CN− NO2− CN− F− 4. Which expression defines the autoionization constant for water, Kw? a. b. c. [H3O+][OH−] [H2O][H3O+] [OH−][H2O] d. e. [H4O2+][O2−] [H2O][H2O] 5. The hydronium ion concentration of a dilute solution of vinegar is 1.45 10−5. What is the pH of this solution? a. b. c. 5.7 −4.8 4.8 d. e. −5.7 7.0 6. What is the concentration of the acetate ion in a 1.00 M CH3COOH solution given that the Ka of CH3COOH is 1.8 10−5? a. b. c. 4.20 10−3 M 0.996 M 1.00 M d. e. 2.84 10−4 M 5.62 10−5 M 7. Boric acid frequently is used as an eyewash to treat eye infections. The pH of a 0.050 M solution of boric acid is 5.28. What is the value of the boric acid ionization constant, Ka? Treat boric acid as a monoprotic acid that dissociates in the following manner: H3BO3 (aq) + H2O (l) a. b. c. 5.25 10−6 5.51 10−10 5.43 10−8 H3O+(aq) + H2BO3- (aq) d. e. 5.79 10−4 5.33 10−12 8. What is the concentration of [OH−] in a 0.20 M solution of ammonia? Kb = 1.8 10−5. NH3(aq) + H2O (l) a. b. c. NH4(aq) + OH-(aq) 3.6 10−6 M 1.8 10−5 M 0.20 M d. e. 1.9 10−3 M 4.2 10−4 M 9. Identify the weakest and strongest acids in the group below. The weakest acid is listed first in the responses. H2O, H2S, H2Se a. H2Se H2S H2O d. H2O H2Se H2S b. H2Se H2O H2S e. H2O H2S H2Se c. H2S H2Se H2O 10. Aqueous solutions of ________ are acidic. a. b. c. NaF CsCN MgCl2 d. e. NH4I KI 11. What is Kb for the conjugate base of an acid having Ka = 8.40 10−4? a. b. c. 2.40 10−12 6.23 10–8 6.17 10−10 d. e. 4.80 10−11 1.19 10−11 12. What is the pH of a 0.35 M solution of sodium azide, Na3N? Ka = 1.9 10−5 for hydrazoic acid, H3N. a. b. c. 11.41 4.87 9.13 d. e. 2.59 12.47 13. Identify all the correct statements about an acid–base buffer solution. I. It can be prepared by combining a strong acid with a salt of its conjugate base. II. It can be prepared by combining a weak acid with a salt of its conjugate base. III. It can be prepared by combining a weak base with its conjugate acid. IV. The pH of a buffer solution does not change when the solution is diluted. V. A buffer solution resists changes in its pH when an acid or base is added to it. a. b. c. I, II, and IV II, III, and V II, III, IV, and V d. e. I, II, IV, and V II, III, and IV 14. Which of the following cannot be mixed together in water to produce a buffer solution? a. b. c. NH3 and HCl HF and KF HClO4 and NaClO4 d. e. HCN and NaOH HNO3 and NaNO3 15. What is the pH of a solution containing 0.30 M lactic acid and 0.50 M of lactate ion (Ka for lactic acid = 1.38 10−4)? a. b. c. 9.92 10.36 3.86 d. e. 4.08 3.64 16. The following titration curve is most likely to be associated with a. b. c. d. the titration of a strong acid with a strong base titrant. the titration of a weak acid with a strong base titrant. the titration of a strong base with a strong acid titrant. the titration of a weak base with a strong acid titrant. 17. A solution of ammonia (0.12 M, 15.00 mL) was titrated with 0.2 M hydrochloric acid (Kb of ammonia = 1.8 10−5). What is the pH at the equivalence point? a. 3.75 d. 8.81 b. 5.19 e. 10.25 c. 7.00 18. Identify the Lewis base in the following reaction: a. b. c. PH3 H+ PH4+ d. e. None of these is a base. All of these are bases. 19. Which of the following represents the correct Ksp expression for the reaction shown below? Al2(SO4)3(s) 2Al3+(aq) + 3SO42−(aq) a. [Al3+]2[SO42−]3 d. b. c. 2[Al3+] + 3[SO42−] e. [Al3+][SO42−] 20. What is the solubility of barium sulfate in a solution containing 0.050 M sodium sulfate? The Ksp value for barium sulfate is 1.1 10−10. a. 7.4 10−6 M d. 2.2 10−9 M −11 b. 5.5 10 M e. 1.1 10−10 M −5 c. 1.0 10 M 21. Calculate the minimum concentration of Mg2+ that must be added to 0.10 M NaF in order to initiate a precipitate of magnesium fluoride. (For MgF2 , Ksp = 6.9 × 10–9.) a. b. c. d. e. 1.4 × 107 M 6.9 × 10–9 M 6.9 × 10–8 M 1.7 × 10–7 M 6.9 × 10–7 M CHM202 Unit 3 Practice Answer Section MULTIPLE CHOICE 1. ANS: C Rationale: Collect and Organize We are given five acids and are asked to find the weak acid among the strong acids. Analyze and Solve Look at each acid’s formula for the strong and weak electrolytes: a. HNO3 – strong electrolyte, strong acid b, H2SO4 – strong electrolyte, strong acid c. H2CO3 – weak electrolyte, weak acid d. HCl – strong electrolyte, strong acid e. HClO4 – strong electrolyte, strong acid Think about it It is necessary to know strong and weak electrolytes to recognize the strength of acids. Carbonate is a weak electrolyte. PTS: 1 2. ANS: C Rationale: Collect and Organize It is implied n the question that the hydrogen carbonate, HCO 3-, ion is acting as an acid. Analyze: The conjugate base of an acid is the species that remains after the proton (hydrogen ion, H +, is donated. Solve: If you remove the only hydrogen from the ion, CO 32- remains. Think about it: There you go! PTS: 1 3. ANS: A Rationale: Collect and Organize Given the strengths of the acids, HF HNO2 HCN Ka = 7.2 10−4 Ka = 4.5 10−4 Ka = 6.2 10−10 determine the relative strengths of their conjugate bases. Analyze: The stronger the base, the weaker the acid, so it really isn’t necessary to calculate the Kb values for each conjugate base. The order is the same as the increasing order of acid strength. Solve: The weakest acid among these is HCN, followed by HNO2 and HF, in that order. Thus, the decreasing order of conjugate base strength is CN-, NO2-, and F-. Think about it: This conforms with the definition of weak base strength. PTS: 1 4. ANS: A Rationale: The mass action expression for the autoionization of water, H2O + H2O H3O+ + OH- is Since [H2O] is ignored, the Kw expression is PTS: 1 5. ANS: C Rationale: Collect and Organize Given: Ka = 1.45 x 10-5, determine pH. Analyze: pH = -log Ka Solve: pH = -log Ka pH = -log (1.45 x 10-5) = -(-4.839) = 4.839 Think about it: From the Ka, we would expect a pH between 4 and 5. PTS: 1 6. ANS: A Rationale: Collect and Organize Given: CH3COOH (aq) + H2O (l) H3O+(aq) + CH3COO-(aq) (dissociation of acetic acid) and Ka = 1.8 x 10-5, determine pH of a 1.00 M solution of CH3COOH. Analyze: We will need to set up a RICE table and solve for the concentration of hydronium ion, [H 3O+] Reaction Initial Change Equilibrium CH3COOH (aq) + [CH3COOH] 1.00 -x 1.00 - x H2O (l) [H2O] N/A N/A N/A H3O+(aq) + [H3O+] 0 +x x CH3COO-(aq) [CH3COO-] 0 +x x Solve: We may ignore x in the denominator since the value of x must be insignificantly small compared to the concentration of the acid, so Think about it: Since this is a weak acid, we should expect a small concentration of its conjugate base. PTS: 1 7. ANS: B Rationale: Collect and Organize Given: H3BO3 (aq) + H2O (l) H3O+(aq) + H2BO3- (aq) pH = 5.28 [H3BO3] = 0.050M Analyze: We must first determine the [H3O+] from the pH. [H3O+] = 10-5.28 = 5.25 x 10-6 We can ignore the value in the denominator because it is insignificant compared to the initial concentration of boric acid. Reaction H3BO3 (aq) + H2O (l) H3O+(aq) + H2BO3- (aq) [H3BO3] [H2O] [H3O+] [H2BO3-] 0.050 N/A 5.25 x 10-6 5.25 x 10-6 Equilibrium Solve: Think about it: In order to produce such a small hydronium ion concentration, the Ka must be very small, and it is. PTS: 1 8. ANS: D Rationale: Collect and Organize Given: NH3(aq) + H2O (l) NH4+(aq) + OH-(aq) [NH3] = 0.20M Kb = 1.8 10−5 Analyze: Create a RICE table for the reaction: Reaction NH3(aq) + H2O (l) NH4+(aq) + OH-(aq) [NH3] [H2O] [NH4+] [OH -] Initial 0.20 N/A 0 0 Change -x N/A +x +x 0.20 - x N/A x x Equilibrium Solve: Think about it: We are only being asked for [OH-], so we are done. The answer is reasonable. PTS: 1 9. ANS: D Rationale: Collect and Organize Given: Three acids with conjugate base anions from the same periodic table group, determine their relative strengths. Analyze: Recall that dissociation depends on the attraction between ions in aqueous solution, i.e., the greater the attraction, the lower the dissociation. Since the strength of acids depends on the extent of acid dissociation, this principle applies. Attraction depends on both the electrical charges and the distance between the charges. In this case, the charge is the same for all anions (they’re in the same PT group), so the inverse relationship of distance will determine the strength. Solve: Now we merely arrange the acids in order of the sizes of their conjugate bases. H2O < H2S < H2Se Think about it: According to Coulomb’s Law, the smallest anion, oxide, would have the greatest attraction and be the weakest acid. PTS: 1 10. ANS: D Rationale: Collect and Organize Given: We are given five salts, NaF – sodium fluoride, CsCN – cesium cyanide, MgCl2 – magnesium chloride, NH4I – ammonium iodide and KI – potassium iodide. We are asked which one is acidic. Analyze: Salts are formed in acid-base neutralization reactions. The stronger of the two, if any, determines whether the salt is acidic or basic. Solve: a, NaF – sodium fluoride – formed from a strong base and a weak acid ?9? basic. b. CsCN – cesium cyanide – formed from a strong base and a weak acid ?9? basic. c. MgCl2 – magnesium chloride – formed from a strong base and a strong acid ?9? neutral. d. NH4I – ammonium iodide – formed from a weak base and a strong acid ?9? acidic. e. KI – potassium iodide – formed from a strong base and a strong acid ?9? neutral. Think about it: We are able to eliminate all but one salt. PTS: 1 11. ANS: E Rationale: Collect and Organize Given: We are given the acid ionization constant, and we are asked to determine its conjugate base’s base ionization constant. Ka = 8.40 10−4 Analyze: From the autoionization of water, we know that Kw = 1.00 x 10-14. From the chemistry of neutralization reactions, we know that Ka x Kb = Kw. Solve: Think about it: The result is reasonable. PTS: 1 12. ANS: C Rationale: Collect and Organize Given: The soluble salt of a strong base and a weak acid is dissolved. The weak acid, hydroazoic acid has Ka = 1.9 10−5. and its concentration is 0.35M. Analyze: The sodium salt dissociates completely in water by NaN3(aq) ?4? Na+(aq) + N3-(aq) and the azide ion hydrolyzes N3-(aq) + H2O (l) HN3 (aq) + OH-(aq) We need the Kb of the conjugate base, N3-: We will need a RICE table: Reaction N3-(aq) + H2O (l) H3N(aq) + OH-(aq) [N3-] [H2O] [HN3] [OH-] Initial 0.35 N/A 0 0 Change -x N/A +x +x Equibrium 0.35 – x N/A x x Solve: since x is insignificant as compared with concentration. Think about it: This is a salt of a strong base and a weak acid, so the high pH is expected. PTS: 1 13. ANS: C Rationale: I. It can be prepared by combining a strong acid with a salt of its conjugate base. - No II. It can be prepared by combining a weak acid with a salt of its conjugate base. – Yes, definition. III. It can be prepared by combining a weak base with its conjugate acid. – Yes, definition. IV. The pH of a buffer solution does not change when the solution is diluted. – Yes, both concentrations will change, ant the pH is computed on the basis of the ratios of the concentration. V. A buffer solution resists changes in its pH when an acid or base is added to it. – Yes, the acid will neutralize added base, and the base will neutralize the acid. PTS: 1 14. ANS: E Rationale: a. NH3 and HCl - No. They do not contain a conjugate acid-base pair. b. HF and KF – Yes. A weak acid and its conjugate base (definition). c. HClO4 and NaClO4 – No. Strong acid. d. HCN and NaOH– No. Strong base. e.HNO3 and NaNO3– No. Strong acid. PTS: 1 15. ANS: D PTS: 1 16. ANS: D PTS: 1 17. ANS: B PTS: 1 18. ANS: A PTS: 1 19. ANS: A PTS: 1 20. ANS: D PTS: 1 21. ANS: E PTS: 1