Li-ion Battery Recycling: Sustainable Material Recovery Process
advertisement

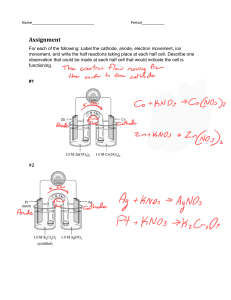
sustainability Article A Sustainable Process for the Recovery of Anode and Cathode Materials Derived from Spent Lithium-Ion Batteries Guangwen Zhang 1 , Zhongxing Du 1 , Yaqun He 1,2, *, Haifeng Wang 1 , Weining Xie 2,3 and Tao Zhang 4 1 2 3 4 * School of Chemical Engineering and Technology, China University of Mining and Technology, No.1 Daxue Road, Xuzhou 221116, China; gwzhang@cumt.edu.cn (G.Z.); 06152238@cumt.edu.cn (Z.D.); whfcumt@cumt.edu.cn (H.W.) Advanced Analysis and Computation Center, China University of Mining and Technology, No.1 Daxue Road, Xuzhou 221116, China; aacc_xwn5718@cumt.edu.cn Jiangsu Huahong Technology Stock Limited Company, Wuxi 214400, China Research Institute of Tsinghua University in Shenzhen, Shenzhen 518057, China; zhangt@tsinghua-sz.org Correspondence: yqhe@cumt.edu.cn; Tel.: +86-516-8359-2928; Fax: +86-516-8399-5026 Received: 15 March 2019; Accepted: 15 April 2019; Published: 20 April 2019 Abstract: The recovery of cathode and anode materials plays an important role in the recycling process of spent lithium-ion batteries (LIBs). Organic binders reduce the liberation efficiency and flotation efficiency of electrode materials derived from spent LIBs. In this study, pyrolysis technology is used to improve the recovery of cathode and anode materials from spent LIBs by removing organic binders. Pyrolysis characteristics of organics in electrode materials are investigated, and on this basis, the effects of pyrolysis parameters on the liberation efficiency of electrode materials are studied. Afterwards, flotation technology is used to separate cathode material from anode material. The results indicate that the optimum liberation efficiency of electrode materials is obtained at a pyrolysis temperature of 500 ◦ C, a pyrolysis time of 15 min and a pyrolysis heating rate of 10 ◦ C/min. At this time, the liberation efficiency of cathode materials is 98.23% and the liberation efficiency of anode materials is 98.89%. Phase characteristics of electrode materials cannot be changed under these pyrolysis conditions. Ultrasonic cleaning was used to remove pyrolytic residues to further improve the flotation efficiency of electrode materials. The cathode material grade was up to 93.89% with a recovery of 96.88% in the flotation process. Keywords: electrode materials; spent lithium-ion battery; pyrolysis; liberation; flotation; recovery 1. Introduction Lithium-ion batteries (LIBs) are widely used as an energy-storage component in various electrical and electronic products, including mobile phones, cameras, laptop computers, and new-energy vehicles, due to their excellent characteristics, such as high energy density, safe handling, and low self-discharge [1,2]. The demand for LIBs is presenting a rapid growth trend [3]; meanwhile, the growth rate is increasing gradually, which means that large amounts of spent LIBs need to be treated because of their limited lifetime [4]. Spent LIBs mainly comprise a metallic shell, membrane separator, cathode materials (LiCoO2 , LiMn2 O4 , LiFePO4 , as well as other lithium metal oxides), aluminum foil, anode materials (graphite), copper foil, and organic electrolytes [5]. The abundant components of spent LIBs indicate that the recycling of valuable metals from spent LIBs is a significant process. From the viewpoint of environmental protection and resource recovery, the recycling of spent LIBs has attracted more and more attention, and some sophisticated processes including hydrometallurgy, Sustainability 2019, 11, 2363; doi:10.3390/su11082363 www.mdpi.com/journal/sustainability Sustainability 2019, 11, 2363 2 of 11 pyrometallurgy, and bio-metallurgy have been proposed [6–8]. It is worth noting that current metallurgy technologies always focus on the recovery of high economic value metals derived from cathode materials, especially Co, Li, Mn, and Ni [9]. Therefore, how to obtain pure cathode materials is the core process that connects the pretreatment process with metallurgy. Manual dismantling is extensively used to obtain cathode materials for the metallurgy process. Each component is obtained using the dismantling process and then cathode is selected as a research target. N-methyl-2-pyrrolidone or other chemical solutions are usually used to remove the organic binder, and then cathode materials are liberated from the aluminum foils [10,11]. By sieving, pure cathode materials are used in subsequent metallurgy processes. However, manual dismantling is harmful to people’s health and limits the industrial recovery efficiency of spent LIBs. At the same time, using a chemical solution to remove the organic binder has some problems, including low liberation efficiency and secondary pollution. In recent years, with the production increase of spent LIBs, mechanical-physical methods have been applied in the pretreatment process for spent LIBs. Multistage crushing can liberate electrode particles from foils, while screening processes are utilized to achieve the separation of electrode materials and foils [12–14]. Flotation technology is then utilized to realize the separation of cathode and anode materials due to their obvious hydrophilic/hydrophobic differences [15–17]. This flowchart is significant for large scale industrial application and the preparation of pure cathode materials for the metallurgy process. Two main problems need to be solved in this process: (a) Electrode particles are attached to foils by organic binder and they are difficult to liberate under only the action of mechanical crushing force, which results in some electrode materials remaining on the foils and decreases the recovery efficiency; (b) electrode materials from this flowchart are still enveloped by electrolyte and organic binder, which will decrease their flotation efficiency because the hydrophilic/hydrophobic differences between cathode materials and anode materials are decreased. Therefore, the removal of organic binder and electrolyte is an essential process in order to obtain high purity cathode materials [18,19]. Some chemical agents, including N-methyl-2-pyrrolidone, dimethylformamide, dimethyl acetamide, and dimethyl sulfoxide, have been utilized to dissolve the organic binder [20,21]. However, chemical dissolution methods characterized by high cost, low efficiency, and potential pollution limit its wide application. A Fenton high-order oxidation process has been used to remove organic binder and residual electrolytes to improve the flotation efficiency of electrode materials. However, Fe2+ was introduced into this reaction, and the Fe element remained on the surface of the electrode material, complicating the subsequent metallurgical process [15,22]. Yu et al. adopted a grinding process to improve the flotation efficiency of electrode materials. Although grinding can improve the flotation efficiency, the organic binders and electrolytes still remain on the particles, resulting in a low cathode material recovery [23]. A roasting method can remove the organic binder and electrolytes adequately [24], but the serious secondary pollution limits its application. Pyrolysis is an effective method that can not only remove organics from electrode materials but also reduce the environmental pollution. A sustainable process for the recovery of cathode and anode materials derived from spent lithium-ion batteries is proposed in this study. The main purpose of this research work is to obtain high purity cathode material to lay the foundation for metallurgy. This research focuses on the following aspects: (a) The pyrolysis characteristics of cathode and anode materials; (b) the facilitation effect of pyrolysis on the liberation of electrode materials; (c) the effects of pyrolysis on the phase properties of electrode materials; and (d) the enhancement of electrode materials’ flotation behavior by pyrolysis. 2. Experimental Section 2.1. Experimental Methods Spent LIBs derived from waste mobile phones were collected, comprising various types of batteries. The main cathode material was LiCoO2 , but there was a small amount of Mn and Ni elements in the cathode material [15]. The anode material was graphite. In this study, the spent LIBs were treated Sustainability 2019, 11, 2363 3 of 11 Sustainability 2019, 11, x FOR PEER REVIEW 3 of 11 following the flowchart shown in Figure 1. They were firstly discharged with 5 wt% NaCl solution for were treated following the flowchart shown in Figure 1. They were firstly discharged with 5 wt% 48 h and then naturally air-dried. The discharged spent LIBs were firstly cut up by a shredder. As a NaCl solution for 48 h and then naturally air-dried. The discharged spent LIBs were firstly cut up by result, the metallic shells were shredded while the membrane separator, electrode sheets, and other a shredder. As a result, the metallic shells were shredded while the membrane separator, electrode components were liberated from each other. After this shredding process, the metallic shell, membrane sheets, and other components were liberated from each other. After this shredding process, the separator, and electrode sheets presented flake shapes, and pneumatic separation was then used metallic shell, membrane separator, and electrode sheets presented flake shapes, and pneumatic to remove the membrane separator and other plastics. Magnetic (for steel shell) or eddy current separation was then used to remove the membrane separator and other plastics. Magnetic (for steel (for aluminum shell) separations were applied to remove metallic shells. The mixture of cathode shell) or eddy current (for aluminum shell) separations were applied to remove metallic shells. The and anode scraps were obtained after the pretreatment. Part of the cathode and anode scraps was mixture of cathode and anode scraps were obtained after the pretreatment. Part of the cathode and directly crushed and sieved. Other electrode mixtures were treated using pyrolysis combined with anode scraps was directly crushed and sieved. Other electrode mixtures were treated using pyrolysis a mechanical-physical process. Afterwards, a flotation process was used to achieve the separation combined with a mechanical-physical process. Afterwards, a flotation process was used to achieve of the cathode and anode materials. The effects of pyrolysis on the liberation efficiency and flotation the separation of the cathode and anode materials. The effects of pyrolysis on the liberation efficiency efficiency of electrode materials were then investigated. and flotation efficiency of electrode materials were then investigated. Figure 1. 1. Experimental Experimental flowchart flowchart of of this this study. study. Figure 2.2. Pyrolysis Experiment 2.2. Pyrolysis Experiment A portion of the cathode and anode scraps were hand-sorted to analyze their pyrolysis A portion of the cathode and anode scraps were hand-sorted to analyze their pyrolysis characteristics. Pyrolysis experiments of cathode and anode mixtures were conducted in a characteristics. Pyrolysis experiments of cathode and anode mixtures were conducted in a controlledcontrolled-atmosphere tube furnace (MXG1200-80, Shanghai Micro-X furnace Co., Ltd., Shanghai, atmosphere tube furnace (MXG1200-80, Shanghai Micro-X furnace Co., Ltd., Shanghai, China), using China), using the pyrolysis system shown in Figure 2. The mixed cathode and anode scraps were the pyrolysis system shown in Figure 2. The mixed cathode and anode scraps were placed into the placed into the tube furnace. Afterwards, the corun-dum tube was filled with nitrogen, with the tube furnace. Afterwards, the corun-dum tube was filled with nitrogen, with the nitrogen flow nitrogen flow remaining at 100 mL/min to carry pyrolysis products away. Pyrolysis parameters were remaining at 100 mL/min to carry pyrolysis products away. Pyrolysis parameters were set using a set using a temperature control system. Pyrolysis products from the organics in electrode materials temperature control system. Pyrolysis products from the organics in electrode materials were taken were taken out of the tube by nitrogen flow, and pyrolysis oils were then collected by condensation, out of the tube by nitrogen flow, and pyrolysis oils were then collected by condensation, while while organic gas was collected and treated by a gas collector system. Deionized water and an NaOH organic gas was collected and treated by a gas collector system. Deionized water and an NaOH solution were used to remove the acidic gas. solution were used to remove the acidic gas. Sustainability 2019, 11, 2363 4 of 11 Sustainability 2019, 11, x FOR PEER REVIEW 4 of 11 Figure 2. Schematic Schematicdiagram diagramofofthe thelaboratory laboratorypyrolysis pyrolysis device: temperature control system, device: (A)(A) temperature control system, (B) (B) tubular furnace, condenser pipe, condensation products collector, deionized water 1, tubular furnace, (C) (C) condenser pipe, (D) (D) condensation products collector, (E) (E) deionized water 1, (F) (F) deionized water 2, (G) NaOH solution, (H) tailing gas drying device. deionized water 2, (G) NaOH solution, (H) tailing gas drying device. 2.3. Flotation Experiment 2.3. Flotation Experiment Flotation technology was used to separate cathode material from anode material, because cathode Flotation technology was used to separate cathode material from anode material, because material is hydrophilic, while anode material is hydrophobic. After flotation, anode material will attach cathode material is hydrophilic, while anode material is hydrophobic. After flotation, anode material to bubbles and be collected in the froth product. Flotation experiments were conducted on electrode will attach to bubbles and be collected in the froth product. Flotation experiments were conducted on materials before and after pyrolysis. Before the flotation experiments, the pyrolytic electrode materials electrode materials before and after pyrolysis. Before the flotation experiments, the pyrolytic were ultrasonically-cleaned to remove the pyrolysis residues. Flotation experiments were conducted electrode materials were ultrasonically-cleaned to remove the pyrolysis residues. Flotation using an XFD-63 flotation machine (Jianfeng Mining Machinery Manufacturing co. LTD, Nanchang, experiments were conducted using an XFD-63 flotation machine (Jianfeng Mining Machinery China) at room temperature with an impeller speed of 1800 rpm, an aeration quantity of 2.0 L/min, Manufacturing co. LTD, Nanchang, China) at room temperature with an impeller speed of 1800 rpm, and a pulp density of 40 g/L. A dilute pulp concentration was used in this study because of the fine size an aeration quantity of 2.0 L/min, and a pulp density of 40 g/L. A dilute pulp concentration was used of the electrode materials. n-Dodecane was used as a collector with a dosage of 300 g/t, while methyl in this study because of the fine size of the electrode materials. n-Dodecane was used as a collector isobutyl carbinol (MIBC) was used as frother with a dosage of 150 g/t. Cathode material concentrate with a dosage of 300 g/t, while methyl isobutyl carbinol (MIBC) was used as frother with a dosage of and anode material concentrate were obtained by filtration and drying. Afterwards, cathode material 150 g/t. Cathode material concentrate and anode material concentrate were obtained by filtration and grade in flotation products was tested by ICP. drying. Afterwards, cathode material grade in flotation products was tested by ICP. 2.4. Analytical Methods 2.4. Analytical Methods The pyrolysis characteristics of cathode and anode materials were analyzed by a The pyrolysis characteristics of cathode and anode materials were analyzed by a Thermogravimetric Analyzer (TGA) (NETZSCH, STA 449-F5, Selb, Germany, testing condition: Thermogravimetric Analyzer (TGA) (NETZSCH, STA 449-F5, Selb, Germany, testing condition: temperature rose from 20 to 700 ◦ C with a heating rate of 10 ◦ C/min; N2 was used as a shielding gas temperature rose from 20 to 700 °C with a heating rate of 10 °C/min; N2 was used as a shielding gas with an airflow velocity of 20 mL/min). An Electro-probe microanalyzer (EPMA) (EPMA-8050G, Kyoto, with an airflow velocity of 20 mL/min). An Electro-probe microanalyzer (EPMA) (EPMA-8050G, Japan) was used to investigate the pyrolysis-enhanced liberation mechanism of electrode materials. Kyoto, Japan) was used to investigate the pyrolysis-enhanced liberation mechanism of electrode Surface chemical states of electrode materials were analyzed using an X-ray photoelectron spectroscopy materials. Surface chemical states of electrode materials were analyzed using an X-ray photoelectron (XPS), (ESCALAB 250Xi, Thermo Fisher Scientific Inc., Waltham, MA, USA). A scanning electron spectroscopy (XPS), (ESCALAB 250Xi, Thermo Fisher Scientific Inc., Waltham, MA, USA). A scanning microscope (SEM), (FEI quanta 250, Hillsboro, Oregon, USA) was used to determine the morphology electron microscope (SEM), (FEI quanta 250, Hillsboro, Oregon, USA) was used to determine the of the electrode material particles before and after pyrolysis. The cathode material grades in flotation morphology of the electrode material particles before and after pyrolysis. The cathode material products were analyzed by inductively coupled plasma-mass spectrometry (ICP-MS) (NWR 213-7900 grades in flotation products were analyzed by inductively coupled plasma-mass spectrometry (ICPICP-MS, Palo Alto, California, USA). X-ray powder diffractometry (XRD) (Bruker D8 advance, Bremen, MS) (NWR 213-7900 ICP-MS, Palo Alto, California, USA). X-ray powder diffractometry (XRD) Germany, database: Inorganic Crystal Structural Database and Powder Diffraction File) was used to (Bruker D8 advance, Bremen, Germany, database: Inorganic Crystal Structural Database and Powder analyze the effects of pyrolysis on the mineral phases. Diffraction File) was used to analyze the effects of pyrolysis on the mineral phases. 3. Results and Discussion 3. Results and Discussion 3.1. TG Analysis of Cathode and Anode Materials 3.1. TG Analysis of Cathode and Anode Materials TG analysis played an important role in guiding the selection of the pyrolysis parameters. Cathode TG analysis played important role inprocessing. guiding theAfterwards, selection ofTG the pyrolysis parameters. and anode materials werean obtained by manual analyses of cathode and Cathode and anode were obtained by manual processing. of anode materials werematerials conducted respectively, with the results shown Afterwards, in Figure 3. TG Theanalyses first main cathode and anode materials were conducted respectively, with the results shown in Figure 3. The first main weight-loss stage appeared at about 100 °C, while the second main weight-loss stage Sustainability 2019, 11, 2363 5 of 11 Sustainability 2019, 11, x FOR PEER REVIEW 5 of 11 weight-loss stage appeared at about 100 ◦ C, while the second main weight-loss stage presented at about 500 ◦at C. about The evaporation of electrolyte caused the first weight-loss second weight-loss presented 500 °C. The evaporation of electrolyte caused thestage. first The weight-loss stage. The was from the decomposition of organic binders that are wrapped on the surface of electrode materials. second weight-loss was from the decomposition of organic binders that are wrapped on the surface On the basis of the TG analysis, cathode and anode materials have similar pyrolysis characteristics, of electrode materials. On the basis of the TG analysis, cathode and anode materials have similar and the mixed pyrolysis of cathode and anode materials is feasible. pyrolysis characteristics, and the mixed pyrolysis of cathode and anode materials is feasible. Figure 3. TG from spent spent LIBs. LIBs. Figure 3. TG curves curves of of cathode cathode and and anode anode materials materials derived derived from 3.2. Crushing Characteristics Characteristics of of Electrode Electrode Scraps 3.2. Crushing Scraps Derived Derived from from Spent Spent LIBs LIBs Cathode and anode anode scraps scrapswere weremanually manuallysorted sortedand andthen then crushed using impact crusher. Cathode and crushed using anan impact crusher. A A sieving process was conducted to obtain different sized fractions, and afterwards, ICPused was sieving process was conducted to obtain fivefive different sized fractions, and afterwards, ICP was used to analyze the element distributions in each fraction the results being given in Table 1. Co, to analyze the element distributions in each fraction size, size, the results being given in Table 1. Co, Ni, Ni, Mn, and Li, derived from cathode materials, and C, derived from anode materials, were mainly Mn, and Li, derived from cathode materials, and C, derived from anode materials, were mainly concentrated in the the−0.2 −0.2mm mmsize sizefraction, fraction, while and foils were mainly concentrated in+1.4 the concentrated in while AlAl and CuCu foils were mainly concentrated in the +1.4 mm size fraction, which demonstrates that electrode scraps have selective crushing properties. mm size fraction, which demonstrates that electrode scraps have selective crushing properties. However, materials were distributed in +1.4 mmmm and and −0.5−0.5 + 0.2+mm size However, some someelements elementsfrom fromelectrode electrode materials were distributed in +1.4 0.2 mm fractions, which indicates that some electrode materials still attach foils to under action organic size fractions, which indicates that some electrode materials still to attach foilsthe under theofaction of binders. It is also worth noting that some electrode materials that were liberated from foils were hard organic binders. It is also worth noting that some electrode materials that were liberated from foils to separate from foils using the sieving process, because they still attached each other under the were hard to separate from foils using the sieving process, because they stilltoattached to each other action of organic binders. Based on this element distribution analysis, the removal of organic binders under the action of organic binders. Based on this element distribution analysis, the removal of is a necessary process to improve the recycling efficiency of electrode materials. organic binders is a necessary process to improve the recycling efficiency of electrode materials. Table inin different sized fractions of crushing products of the of raw electrode scraps. Table1.1.Element Elementdistribution distribution different sized fractions of crushing products the raw electrode scraps. Cathode Anode Size Fraction (mm) Cathode Anode Co Ni Mn Li Al Cu C Size Fraction (mm) Mn +1.4 5.79 Co 5.80 Ni 5.72 5.72 −1.4 + 0.71+1.4 1.06 5.790.99 5.80 1.03 −0.71 +−1.4 0.5 + 0.71 0.29 1.060.31 0.99 0.26 1.03 −0.5 +−0.71 0.2 + 0.5 2.29 0.292.09 0.31 2.63 0.26 −0.2 90.57 90.81 90.36 Li 5.55 5.55 1.01 0.28 1.01 1.95 0.28 91.21 Al 80.45 80.45 16.17 0.60 16.17 2.09 0.60 0.69 −0.5 + 0.2 2.29 2.09 2.63 1.95 2.09 −0.2 90.57 90.81 90.36 91.21 0.69 3.3. Facilitation Effect of Pyrolysis on the Liberation of Electrode Materials Cu84.01 C 2.62 84.01 13.20 2.62 0.26 0.61 0.26 0.16 13.20 0.611.53 0.16 6.70 0.65 90.26 1.53 6.70 0.65 90.26 3.3. Facilitation Effect of Pyrolysis on theto Liberation Electrode Materials Cathode materials were adopted analyze of the facilitation effect of pyrolysis on the liberation of electrode materials. Before and after pyrolysis, electrode materialseffect wereof first embedded in self-curing Cathode materials were adopted to analyze the facilitation pyrolysis on the liberation of electrode materials. Before and after pyrolysis, electrode materials were first embedded in selfcuring resin. After polishing, a smooth section of electrode materials was exposed. The EPMA images are shown in Figure 4. As we can see from Figures 4a–c, the electrode particles were tightly adhered Sustainability 2019, 11, 2363 6 of 11 Sustainability 2019, 11, x FOR PEER REVIEW 6 of 11 resin. After polishing, a smooth section of electrode materials was exposed. The EPMA images are shown Figure Asorganic we can binder. see fromThe Figure the electrode particles were tightly adhered to each to eachinother by4.the gaps4a–c, between electrode materials were filled with organic other by Electrode the organic binder. The gaps to between materials were filled withFigure organic binders. materials are hard liberateelectrode using only mechanical crushing. 4dbinders. shows Electrode materials are hard to liberate using only mechanical crushing. Figure 4d shows that organic that organic binders were decomposed after pyrolysis treatment. Pyrolysis residues are porous and binders wereby decomposed pyrolysis treatment. Pyrolysisthe residues are porous and easy crush by easy crush mechanicalafter crushing, which can improve liberation efficiency of electrode mechanical materials. crushing, which can improve the liberation efficiency of electrode materials. Figure 4. EPMA analysis of cathode materials: (a–c) before pyrolysis, (d) after pyrolysis. A pyrolysis treatment liberation A pyrolysis treatment combined combined with with mechanical mechanical crushing crushing was was applied applied to to achieve achieve the the liberation between After crushing, crushing, aa sieving sieving process process was was used used to to obtain between electrode electrode materials materials and and foils. foils. After obtain electrode electrode materials with a size of −0.2 mm. The effects of the pyrolysis parameters on the liberation efficiency of materials with a size of −0.2 mm. The effects of the pyrolysis parameters on the liberation efficiency the cathode and anode materials were investigated. The crushing parameters remained unchanged of the cathode and anode materials were investigated. The crushing parameters remained unchanged in The liberation liberation efficiencies efficiencies of of the the cathode in every every crushing crushing experiment. experiment. The cathode and and anode anode materials materials with with different pyrolysis parameters are given in Figure 5. different pyrolysis parameters are given in Figure 5. The played the the mostmost important role inrole the in liberation process of the electrode The pyrolysis pyrolysistemperature temperature played important the liberation process of the materials. The effectThe of the pyrolysis temperature on the liberation efficiency efficiency of the electrode materials electrode materials. effect of the pyrolysis temperature on the liberation of the electrode ◦ C/min. As the was evaluated at a pyrolysis time of 15 min andmin a pyrolysis heatingheating rate ofrate 10 of materials was evaluated at a pyrolysis time of 15 and a pyrolysis 10 °C/min. As pyrolysis temperature rose, the liberation efficiency first increased and thenand decreased. The optimum the pyrolysis temperature rose, the liberation efficiency first increased then decreased. The ◦ C for the anode material. However, pyrolysis of the cathode material was 500 ◦ Cwas and500 550°C optimum temperature pyrolysis temperature of the cathode material and 550 °C for the anode material. the pyrolysis had little had effectlittle on the liberation of the anode at 450 ◦ C. Under However, the temperature pyrolysis temperature effect on the liberation of thematerial anode material at 450 °C. the higher pyrolysis temperature, the liberation of cathode material declined sharply because of the Under the higher pyrolysis temperature, the liberation of cathode material declined sharply because sintering of organic binders. binders. Differences in organicinbinders differinginliberation of the sintering of organic Differences organicresulted bindersinresulted differingefficiencies liberation between the cathode material and the anode material. The optimum pyrolysis temperature of the efficiencies between the cathode material and the anode material. The optimum pyrolysis ◦ mixed electrode materials was intended to be was 500 intended C. temperature of the mixed electrode materials to be 500 °C. The effect of the pyrolysis time on the liberation efficiency of electrode investigated effect of the pyrolysis time on the liberation efficiency of materials electrodewas materials was ◦ ◦ at a pyrolysis at temperature oftemperature 500 C with aof pyrolysis rate of 10 heating C/min. The efficiency investigated a pyrolysis 500 °C heating with a pyrolysis rateliberation of 10 °C/min. The of cathodeefficiency materialsofchanged significantly as thesignificantly pyrolysis heating rate changed. liberation cathode materials changed as the pyrolysis heatingThe rateoptimum changed. pyrolysis time of cathodetime materials was 15materials min. At the time,At thethe pyrolysis timethe hadpyrolysis little effect on The optimum pyrolysis of cathode wassame 15 min. same time, time the of anode longer materials. pyrolysis time the sintering phenomenon hadliberation little effect on thematerials. liberation The of anode The aggravated longer pyrolysis time aggravated the sintering phenomenon of the organic binder, decreasing the liberation efficiency of the electrode materials. The optimum pyrolysis time of the mixed electrode materials was 15 min. Sustainability 2019, 11, 2363 7 of 11 of the organic binder, decreasing the liberation efficiency of the electrode materials. The optimum pyrolysis time of the mixed electrode materials was 15 min. Sustainability 2019, 11, x FOR PEER REVIEW 7 of 11 Figure5.5.Effects Effectsofofpyrolysis pyrolysisparameters parameterson onthe theliberation liberationefficiency efficiencyof ofelectrode electrodematerials: materials: (a) (a) effect effect of Figure of pyrolysis temperature liberation cathodematerials; materials; (b) (b) effect onon pyrolysis temperature on on thethe liberation ofofcathode effect of ofpyrolysis pyrolysistemperature temperature the liberation of anode materials; (c) effect of pyrolysis time on the liberation of cathode materials; (d) the liberation of anode materials; (c) effect of pyrolysis time on the liberation of cathode materials; ofof anode materials; (e)(e) effect ofof pyrolysis heating rate onon thethe (d)effect effectofofpyrolysis pyrolysistime timeononthe theliberation liberation anode materials; effect pyrolysis heating rate liberation of cathode materials; (f) effect of pyrolysis heating rate on the liberation of anode materials. liberation of cathode materials; (f) effect of pyrolysis heating rate on the liberation of anode materials. Pyrolysis-crushingexperiments experimentswere wereconducted conductedtotoevaluate evaluatethe the effect pyrolysis heating Pyrolysis-crushing effect ofof thethe pyrolysis heating rate rate on the liberation efficiency of electrode materials at a pyrolysis temperature of 500 °C and a ◦ on the liberation efficiency of electrode materials at a pyrolysis temperature of 500 C and a pyrolysis pyrolysis timeThe of 15 min. Theefficiency liberationofefficiency electrodedecreased materials as decreased as theheating pyrolysis time of 15 min. liberation electrodeofmaterials the pyrolysis rate heating rate rose. The fast heating rate resulted in the inadequate decomposition of the organic rose. The fast heating rate resulted in the inadequate decomposition of the organic binder, and then binder, and then decreased the liberation efficiency. The effect of the pyrolysis heating rate on the decreased the liberation efficiency. The effect of the pyrolysis heating rate on the liberation efficiency liberation efficiency of electrode materials was not obvious. There was no obvious change in the of electrode materials was not obvious. There was no obvious change in the liberation efficiency of liberation efficiency of electrode materials when the pyrolysis heating rate improved from 5 °C/min electrode materials when the pyrolysis heating rate improved from 5 ◦ C/min to 10 ◦ C/min, but the time to 10 °C/min, but the time was saved by half. From the viewpoint of energy saving, the optimum was saved by half. From the viewpoint of energy saving, the optimum pyrolysis heating rate was set at pyrolysis heating rate was set at 10 °C/min. 10 ◦ C/min. The optimum pyrolysis parameters for the liberation of electrode materials were determined to be a pyrolysis temperature of 500 °C, a pyrolysis time of 15 min, and a pyrolysis heating rate of 10 °C/min. Electrode scraps were pyrolysis-treated at the optimum parameters and were mechanically crushed. At this time, the liberation efficiency of cathode materials was 98.23% and the liberation Sustainability 2019, 11, 2363 8 of 11 The optimum pyrolysis parameters for the liberation of electrode materials were determined to be a pyrolysis temperature of 500 ◦ C, a pyrolysis time of 15 min, and a pyrolysis heating rate of 10 ◦ C/min. Electrode scraps were pyrolysis-treated at the optimum parameters and were mechanically crushed. x FOR PEER REVIEW of cathode materials was 98.23% and the liberation efficiency 8 of 11 AtSustainability this time,2019, the 11, liberation efficiency of anode materials was 98.89%. Afterwards, the element distribution of different sized fractions of efficiency of anode materials was 98.89%. Afterwards, the element distribution of different sized crushing products of the pyrolytic electrode scraps was analyzed, and the results are shown in Table 2. fractions of crushing products of the pyrolytic electrode scraps was analyzed, and the results are From the results, we can see that the contents of Co, Ni, Mn, Li, and C in coarse fractions decreased shown in Table 2. From the results, we can see that the contents of Co, Ni, Mn, Li, and C in coarse after pyrolysis treatment, which indicates that the electrode materials had been adequately liberated fractions decreased after pyrolysis treatment, which indicates that the electrode materials had been from the foils and that the liberation between electrode materials was more adequate. adequately liberated from the foils and that the liberation between electrode materials was more adequate. Table 2. Element distribution in different sized fractions of crushing products from the pyrolytic electrode scraps. Table 2. Element distribution in different sized fractions of crushing products from the pyrolytic electrode scraps. Cathode Anode Size Fraction (mm) Cathode Anode Co Ni Mn Li Al Cu C Size Fraction (mm) Co 0.73 Ni 0.59 Mn Li Al83.76 Cu 88.63C 0.55 0.67 0.630.37 0.73 0.31 0.59 0.55 0.39 83.76 11.2188.63 8.930.67 0.03 0.08 11.21 1.03 8.93 0.550.03 0.06 0.360.11 0.37 0.09 0.31 0.39 0.42 0.62 0.95 2.79 0.09 0.11 0.09 0.08 1.03 0.55 1.060.06 0.53 98.37 98.39 98.03 1.21 0.79 0.42 0.62 0.95 2.79 1.06 0.830.53 98.71 −0.2 98.13 98.37 98.39 98.03 1.21 0.83 98.71 3.4. Effects of Pyrolysis on the Phase Properties of Electrode Materials 3.4. Effects of Pyrolysis on the Phase Properties of Electrode Materials XRD was used to evaluate the effects of pyrolysis on the phase properties of electrode materials. to evaluate the◦ C, effects of pyrolysis on the phase properties of electrode Before XRD and was afterused pyrolysis at 500 electrode materials were tested by XRD, and theirmaterials. results are Before and after pyrolysis at 500 °C, electrode materials were tested by XRD, and their results are 2 , shown in Figure 6. The raw electrode material phase indicated that the cathode material was LiCoO ◦ shown in Figure 6. The raw electrode material phase indicated that the cathode material was LiCoO 2, and the anode material was graphite. After pyrolysis treatment at 500 C, no new material peaks were and theby anode was graphite. After pyrolysis treatment at 500 °C, no new material peaks were detected XRDmaterial in electrode materials, which indicated that phase properties of electrode materials detected by XRD in electrode materials, which indicated◦ that phase properties of electrode materials ◦ were not changed at the pyrolysis temperature of 500 C. The XRD results demonstrate that 500 C were not changed at the pyrolysis temperature of 500 °C. The XRD results demonstrate that 500 °C is is an optimum pyrolysis temperature and it not only can achieve adequate liberation of electrode an optimum pyrolysis temperature and it not only can achieve adequate liberation of electrode materials, but also does not change their phase properties. materials, but also does not change their phase properties. +1.4 0.63 −1.4 + 0.71 +1.4 0.36 −0.71 + −1.4 0.5 + 0.71 0.09 −0.5 + 0.2 −0.71 + 0.5 0.79 −0.2 −0.5 + 0.2 98.13 Figure6.6.XRD XRDresults results of of electrode electrode materials treatment. Figure materialsbefore beforeand andafter afterpyrolysis pyrolysis treatment. 3.5. Enhancement Flotation Behavior of Electrode Materials by Pyrolysis XPS was used to analyze the surface properties of the pyrolytic electrode materials, and the results are given in Figure 7a and Table 3. C–CF and C–CF groups that were from the pyrolysis products (fluorobenzene) were detected on the surface of the pyrolytic electrode materials, and the Sustainability 2019, 11, 2363 9 of 11 3.5. Enhancement Flotation Behavior of Electrode Materials by Pyrolysis XPS was used to analyze the surface properties of the pyrolytic electrode materials, and the results are given Figure 7a and 3. C–CF and C–CF groups that were from the pyrolysis products Sustainabilityin 2019, 11, x FOR PEERTable REVIEW 9 of 11 (fluorobenzene) were detected on the surface of the pyrolytic electrode materials, and the contents of C–CF andofC–CF weregroups 10.89%.were In addition, carbon was also detected surfaceon of contents C–CFgroups and C–CF 10.89%. pyrolytic In addition, pyrolytic carbon was on alsothe detected the pyrolytic materials, the relative content of pyrolytic carbon was 7.98%. Thewas pyrolysis surface ofelectrode the pyrolytic electrode materials, the relative content of pyrolytic carbon 7.98%. residues were hydrophobic, some cathode easily attach to bubbles and collect in the The pyrolysis residues weremaking hydrophobic, makingmaterials some cathode materials easily attach to bubbles froth product, which reduced its which flotation efficiency. and collect in the froth product, reduced its flotation efficiency. In order to improve the separation efficiency of cathode and anode material, ultrasonic cleaning was used to remove the pyrolysis residues. Figure 7b and Table 3 show the XPS analysis of pyrolytic electrode cleaning. The contents of C–CF and C–CF groups decreased to 2.18%, electrodematerials materialsafter afterultrasonic ultrasonic cleaning. The contents of C–CF and C–CF groups decreased to while content pyrolytic carbon decreased 4.59%. After ultrasonic some hydrophobic 2.18%,the while the of content of pyrolytic carbon to decreased to 4.59%. Aftercleaning, ultrasonic cleaning, some pyrolysis residues were residues removed.were Theremoved. hydrophily of hydrophily cathode material improved, enhancing the hydrophobic pyrolysis The of cathode material improved, flotation behavior of cathode and of anode materials. enhancing the flotation behavior cathode and anode materials. Figure 7. 7. C C 1s 1s XPS XPS spectrums spectrums of of electrode electrode materials materials before before and and after after pyrolysis: pyrolysis: (a) (a) before before ultrasonic ultrasonic Figure cleaning, (b) after ultrasonic cleaning. cleaning, (b) after ultrasonic cleaning. Table 3. Chemical states Table 3. Chemical states of of carbon carbon in in the the surface surface of of electrode electrode materials materials before before and and after after pyrolysis. pyrolysis. Pyrolytic Electrode Materials Pyrolysis-Ultrasonic Cleaning ElectrodeElectrode Materials Pyrolysis-Ultrasonic Cleaning Pyrolytic Electrode MaterialsAtomic (%) Components BE (eV) FWHM (eV) Components Pyrolytic BE 283.97 Components carbon (eV) Graphite 284.30 Pyrolytic C–C 284.80 283.97 C–H 285.71 carbon C–CF 286.00 Graphite 284.30 O–C–O 287.14 C–C 284.80 C–CF 288.10 CF 289.40 C–H 285.71 C–F 291.02 Pyrolytic FWHM 2206.17 283.97 BE 0.95 Components Area carbon (eV) (eV) Graphite 284.30 27,161.01 0.55 Pyrolytic C–C 284.80 7791.765 0.75 283.97 3607.48 0.95 2206.17 C–H 1.12 carbon 285.71 C–CF 286.00 589.84 0.93 Graphite 287.14 284.30 1491.15 27,161.01 1.09 0.55 O–C–O C–C 284.80 938.791 7791.765 0.75 C–CF 288.10 1.15 CF 289.40 1.23 C–H 285.71 1230.962 3607.48 1.12 C–F 291.02 3001.55 1.88 FWHM 0.87 (eV) 0.57 0.93 0.87 1.00 1.21 0.57 0.99 0.93 1.01 1.25 1.00 1.85 Atomic 2058.53 (%) 8442.1 5574.71 2058.53 350.76 1803.41 BE (eV) FWHM (eV) Materials Area 8442.1 734.85 5574.71 489.02 619.15 350.76 975.02 C–CF 286.00 1.21 1803.41 C–CF 286.00 589.84 0.93 O–C–O 287.14 0.99 734.85 O–C–O 287.14 1491.15 1.09 Flotation and the 1.15 results C–CF technology 288.10 was used 1.01 to separate 489.02anode material C–CF from cathode 288.10 material 938.791 were shown 4. The cathode grade in the cathode material concentrate was increased CF in Table 289.40 1.25 material 619.15 CF 289.40 1230.962 1.23 to 93.89%, while it was only 2.09% in anode material concentrate. The cathode material recovery C–F 291.02 1.85 975.02 C–F 291.02 3001.55 1.88 increased to 96.88%, which indicates a better flotation efficiency was obtained than in the previous studyFlotation [23]. Therefore, highwas separation is beneficial forfrom the subsequent purification. technology used to efficiency separate anode material cathode material and the results In this recycling process, organics in electrode materials were removed using pyrolysis were shown in Table 4. The cathode material grade in the cathode material concentrate wastechnology. increased At the samewhile time, it thewas pyrolysis products were collected reduce the The environmental pollution of the to 93.89%, only 2.09% in anode material to concentrate. cathode material recovery process. After pyrolysis, the liberation efficiency and flotation efficiency of electrode materials were increased to 96.88%, which indicates a better flotation efficiency was obtained than in the previous study [23]. Therefore, high separation efficiency is beneficial for the subsequent purification. In this recycling process, organics in electrode materials were removed using pyrolysis technology. At the same time, the pyrolysis products were collected to reduce the environmental pollution of the process. After pyrolysis, the liberation efficiency and flotation efficiency of electrode materials were improved, which is advantageous to the subsequent treating process. This research Sustainability 2019, 11, 2363 10 of 11 improved, which is advantageous to the subsequent treating process. This research may provide an alternative flowchart for recycling spent LIBs. Table 4. Flotation results of pyrolysis-ultrasonic cleaning electrode materials. Flotation Products Cathode Material Grade (%) Yield (%) Anode material concentrate Cathode material concentrate 2.09 93.89 30.87 69.13 Cathode material recovery 96.88 4. Conclusions Pyrolysis technology was used to improve the recovery of cathode and anode materials from spent LIBs. Pyrolysis can remove organic binders that are wrapped on the surface of electrode materials, improving their liberation efficiency and flotation efficiency. The optimum pyrolysis parameters for the liberation of electrode materials were determined to be a pyrolysis temperature of 500 ◦ C, a pyrolysis time of 15 min, and a pyrolysis heating rate of 10 ◦ C/min. At this time, the liberation efficiency of cathode materials was 98.23% and the liberation efficiency of anode materials was 98.89%. Pyrolysis residues were further removed using ultrasonic cleaning. After this treatment, the flotation efficiency of electrode materials was clearly improved. The cathode material grade increased to 93.89% with a recovery of 96.88%. This research work provides an alternative method for recycling cathode and anode materials from spent LIBs. Author Contributions: In this research, G.Z. is responsible for data processing and paper writing while Y.H. is in charge of directing the whole research work. Z.D. and T.Z. are in charge of carrying out the experiments. H.W. and W.X. are responsible for test analysis. Funding: This research was funded by the National Natural Science Foundation of China (No. 51574234 and No. 51674257), the National College Students’ innovative and entrepreneurial training program (No.201710290029) and Jiangsu Planned Projects for Postdoctoral Research Funds (1701038C). Guangwen Zhang also appreciates the support of Shanghai Tongji Gao Tingyao Environmental Science and Technology Development Foundation. Conflicts of Interest: The authors declare no conflict of interest. References 1. 2. 3. 4. 5. 6. 7. 8. Zeng, X.; Li, J.; Liu, L. Solving spent lithium-ion battery problems in China: Opportunities and challenges. Renew. Sustain. Energy Rev. 2015, 52, 1759–1767. [CrossRef] Bertuol, D.A.; Toniasso, C.; Jimenez, B.M.; Meili, L.; Dotto, G.L.; Tanabe, E.H.; Aguiar, M.L. Application of spouted bed elutriation in the recycling of lithium ion batteries. J. Power Sources 2015, 275, 627–632. [CrossRef] Mo, J.; Jeon, W. The impact of electric vehicle demand and battery recycling on price dynamics of lithium-ion battery cathode materials: A vector error correction model (VECM) analysis. Sustainability 2018, 10, 2870. [CrossRef] Huang, M.; Li, J. The shortest path problems in battery-electric vehicle dispatching with battery renewal. Sustainability 2016, 8, 607. [CrossRef] Xiao, J.; Li, J.; Xu, Z. Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy. J. Hazard. Mater. 2017, 338, 124–131. [CrossRef] Porvali, A.; Aaltonen, M.; Ojanen, S.; Velazquez-Martinez, O.; Eronen, E.; Liu, F.; Wilson, B.P.; Serna-Guerrero, R.; Lundström, M. Mechanical and hydrometallurgical processes in HCl media for the recycling of valuable metals from Li-ion battery waste. Resour. Conserv. Recycl. 2019, 142, 257–266. [CrossRef] Li, J.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2 /graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [CrossRef] Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus Niger. J. Power Sources 2016, 320, 257–266. [CrossRef] Sustainability 2019, 11, 2363 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 11 of 11 Zeng, X.; Li, J.; Singh, N. Recycling of spent lithium-ion battery: A critical review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [CrossRef] Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co. Hydrometallurgy 2016, 161, 54–57. [CrossRef] Xin, Y.; Guo, X.; Chen, S.; Wang, J.; Wu, F.; Xin, B. Bioleaching of valuable metals Li, Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery. J. Clean. Prod. 2016, 116, 249–258. [CrossRef] Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [CrossRef] Zhang, T.; He, Y.; Ge, L.; Fu, R.; Zhang, X.; Huang, Y. Characteristics of wet and dry crushing methods in the recycling process of spent lithium-ion batteries. J. Power Sources 2013, 240, 766–771. [CrossRef] Zhang, T.; He, Y.; Wang, F.; Ge, L.; Zhu, X.; Li, H. Chemical and process mineralogical characterizations of spent lithium-ion batteries: An approach by multi-analytical techniques. Waste Manag. 2014, 34, 1051–1058. [CrossRef] [PubMed] Yu, J.; He, Y.; Li, H.; Xie, W.; Zhang, T. Effect of the secondary product of semi-solid phase Fenton on the flotability of electrode material from spent lithium-ion battery. Powder Technol. 2017, 315, 139–146. [CrossRef] Zhu, X.; Nie, C.; Zhang, H.; Lyu, X.; Qiu, J.; Li, L. Recovery of metals in waste printed circuit boards by flotation technology with soap collector prepared by waste oil through saponification. Waste Mang. 2019, 89, 21–26. [CrossRef] Zhang, T.; He, Y.; Wang, F.; Li, H.; Duan, C.; Wu, C. Surface analysis of cobalt-enriched crushed products of spent lithium-ion batteries by X-ray photoelectron spectroscopy. Sep. Purif. Technol. 2014, 138, 21–27. [CrossRef] Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhang, T.; Xie, W.; Zhu, X. Enhancement in liberation of electrode materials derived from spent lithium-ion battery by pyrolysis. J. Clean. Prod. 2018, 199, 62–68. [CrossRef] Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhu, X. Pyrolysis-ultrasonic-assisted flotation technology for recovering graphite and LiCoO2 from spent Lithium-ion battery. ACS Sustain. Chem. Eng. 2018, 6, 10896–10904. [CrossRef] Wang, M.; Zhang, C.; Zhang, F. Recycling of spent lithium-ion battery with polyvinyl chloride by mechanochemical process. Waste Manag. 2017, 67, 232–239. [CrossRef] [PubMed] Song, X.; Hu, T.; Liang, C.; Long, H.L.; Zhou, L. Direct regeneration of cathode materials from spent lithium iron phosphate batteries using a solid phase sintering method. RSC Adv. 2017, 7, 4783–4790. [CrossRef] He, Y.; Zhang, T.; Wang, F.; Zhang, G.; Zhang, W.; Wang, J. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by Fenton reagent-assisted flotation. J. Clean. Prod. 2018, 143, 319–325. [CrossRef] Yu, J.; He, Y.; Ge, Z.; Li, H.; Xie, W.; Wang, S. A promising physical method for recovery of LiCoO2 and graphite from spent lithium-ion batteries: Grinding flotation. Sep. Purif. Technol. 2018, 190, 45–52. [CrossRef] Wang, F.; Zhang, T.; He, Y.; Zhao, Y.; Wang, S.; Zhang, G.; Zhang, Y.; Feng, Y. Recovery of valuable materials from spent lithium-ion batteries by mechanical separation and thermal treatment. J. Clean. Prod. 2018, 185, 646–652. [CrossRef] © 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).