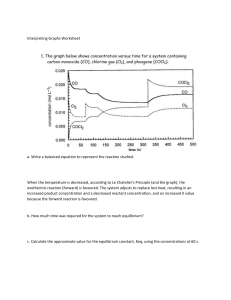
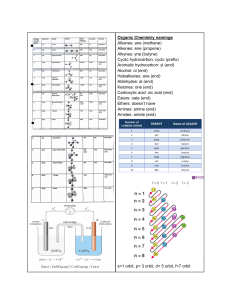
Keq Hand-In WORKSHEET Remember to figure out what type of Keq problem it is before starting 1. A reaction vessel with a capacity of 1.0 litre, in which the following reaction : SO2 (g) + NO2 (g) <===> SO3 (g) + NO (g) had reached a state of equilibrium, was found to contain 0.40 moles of SO3 , 0.30 moles of NO, 0.15 moles of NO2 , and 0.20 moles of SO2. Calculate the equilibrium constant for this reaction. [SO3] = 0.40mol/1.0L = 0.40M [NO] = 0.30mol/1.0L = 0.30M [NO2] = 0.15mol/1.0L = 0.15M [SO2] = 0.20mol/1.0L = 0.20M Keq = [𝑆𝑂3][𝑁𝑂] = [𝑁𝑂2][𝑆𝑂2] (0.40)(0.30) (0.15)(0.20) = 0.12 0.03 = 4.0 2. Given the equilibrium 2 A(g) + B(g) <===> C(g) + 3 D(g) and the concentrations [A] = 2.0 M, [B] = 0.50 M, [C] = 0.25 M, [D] = 1.5 M, calculate the value for the equilibrium constant. Keq = 3 3 [𝐶][𝐷] = 2 [𝐴] [𝐵] (0.25)(1.5) 2 (2.0) (0.50) = 0.84375 2 = 0. 421875 = 0.42 3. Consider the following equation H2 (g) + I2 (g) <===> 2 HI(g) Calculate Keq if at 300oK the concentrations are [H2] = 0.40 M, [I2] = 0.45 M ; [HI] = 0.30 M Keq = 2 2 [𝐻𝐼] [𝐻2][𝐼2] = (0.30) (0.40)(0.45) = 4. 0.09 0.18 = 0.50 The equilibrium constant for the formation of ammonia by the reaction: N2 (g) + 3 H2 (g) <===> 2 NH3 (g) is 2.0 at a certain temperature. If the equilibrium concentration of N2 in a mixture is 0.50 M and H2 is 2.0 M, determine the concentration of ammonia. 2 Keq = [𝑁𝐻3] 3 [𝑁2][𝐻2] 3 𝐾𝑒𝑞[𝑁2][𝐻2] = [NH3] 3 (2. 0)(0. 50)(2. 0) = [NH3] [NH3] = 2.828427125 [NH3] = 2.8 M At 2000oK, a mixture of H2, S2, and H2S rapidly reaches a state of equilibrium represented by the equation : 2 H2S (g) <===> 2 H 2(g) + S2 (g) An analysis of the equilibrium mixture in a 2.00 litre flask reveals the presence of 2.00 moles of H2S and 1.00 mole of H2. If the Keq for this reaction at this temperature is 1.62 x 10-2, how many moles of S2 can we expect to find in this flask ? 5. [H2S] = 2.00mol/2.00L = 1.00M [H2] = 1.00mol/2.00L = 0.500M 2 [𝐻2] [𝑆2] Keq = 2 [𝐻2𝑆] 2 [S2] = [S2] = 𝐾𝑒𝑞[𝐻2𝑆] 2 [𝐻2] −2 2 (1.62 · 10 )(1.00) 2 (0.500) [S2] = 0.0648 M ( 0.0648𝑚𝑜𝑙 𝑆2 1𝐿 )(2. 00𝐿) = 0.130 mol S2 6. The equilibrium constant for the formation of CO2 by the reaction 2 CO (g) + O2 (g) <===> 2 CO2 (g) is 1.0 x 104. If, at equilibrium, the concentration of CO2 is 3.0 M and the concentration of O2 is 9.0 M, calculate the concentration of CO. 2 Keq = [𝐶𝑂2] 2 [𝐶𝑂] [𝑂2] 2 [𝐶𝑂] = [CO] = [𝐶𝑂2] 𝐾𝑒𝑞[𝑂2] 2 (3.0) 4 (1.0 · 10 )(9.0) [CO] = 0.010 M 7. A sample containing 0.800 moles of POCl3 is enclosed in a 0.500 litre vessel at a certain temperature. When the equilibrium for the dissociation reaction: POCl3 (g) <===> POCl (g) + Cl2 (g) is attained, it is found that the vessel contains 0.259 moles of Cl2. Calculate the equilibrium constant. [POCl3] = 0.800mol/0.500L = 1.60M [Cl2] = 0.259mol/0.500L = 0.518M POCl3 (g) ⇌ POCl (g) + Cl2 (g) INITIAL 1.60 0 0 CHANGE -0.518 +0.518 +0.518 EQUIL. 1.082 0.518 0.518 Keq = Keq = [𝑃𝑂𝐶𝑙][𝐶𝑙2] [𝑃𝑂𝐶𝑙3] 2 (0.518) (1.082) Keq = 0.2479889094 Keq = 0.248 8. The brown gas NO2 on cooling is converted into the colourless gas N2O4 as described by the equation: 2 NO2(g) <===> N2O4(g) If the original concentration of NO2 is 0.90 M, and at equilibrium its concentration is only 0.26 M, what is the equilibrium constant for the reaction ? 2 NO2 (g) ⇌ N2O4 (g) INITIAL 0.90 0 CHANGE -0.64 +0.32 EQUIL. 0.26 0.32 Keq = Keq = [𝑁2𝑂4] 2 [𝑁𝑂2] (0.32) 2 (0.26) Keq = 4.733727811 Keq = 4.7 9. SO3 (g) + NO (g) <====> NO2 (g) + SO2 (g) If 0.300 mol of SO3 and 0.300 mol NO were placed in a 1.00 L flask and allowed to react, what would be the equilibrium concentration of each gas ? (Keq = 0.500) [SO3] = 0.300mol/1.00L = 0.300M [NO] = 0.300mol/1.00L = 0.300M SO3 (g) + NO (g) ⇌ NO2 (g) + SO2 (g) INITIAL 0.300 0.300 0 0 CHANGE -x -x +x +x 0.300 - x 0.300 - x x x EQUIL. [𝑁𝑂2][𝑆𝑂2] Keq = 0.500 = 0.500 = [𝑆𝑂3][𝑁𝑂] 2 = 𝑥 2 (0.300 − 𝑥) 2 𝑥 2 (0.300 − 𝑥) 0. 500 = 𝑥 (0.300 − 𝑥) 0. 500(0.300 - x) = x 0. 500(0.300) - 0. 500x = x 0. 500(0.300) = (1 + 0. 500)x 0.500(0.300) (1 + 0.500) = 𝑥 x = 0.1242640687 EQUILIBRIUM CONCENTRATIONS: [SO3] = [NO] = 0.300 - x = 0.300 - 0.1242640687 = 0.176 M [NO2] = [SO2] = x = 0.124 M 10. 2 HCl (g) <====> H2 (g) + Cl2 (g) If 2.00 mol HCl are placed in a 5.00 L flask and allowed to come to the equilibrium shown above, what will the equilibrium [H2] be ? (Keq = 3.2 x 10-4) [HCl] = 2.00mol/5.00L = 0.400M 2 HCl (g) ⇌ H2 (g) + Cl2 (g) INITIAL 0.400 0 0 CHANGE -2x +x +x 0.400 - 2x x x EQUIL. [𝐻2][𝐶𝑙2] Keq = 3.2 x 10-4 = −4 3. 2 · 10 = 2 [𝐻𝐶𝑙] 2 = 𝑥 2 (0.400 − 2𝑥) 𝑥 (0.400 − 𝑥) −4 3. 2 · 10 (0. 400 − 2𝑥) = 𝑥 −4 −4 3. 2 · 10 (0. 400) − 2 3. 2 · 10 𝑥 = 𝑥 −4 −4 3. 2 · 10 (0. 400) = (1 + 2 3. 2 · 10 )𝑥 −4 𝑥= 3.2·10 (0.400) −4 (1+2 3.2·10 ) x = 0.0069082601 EQUILIBRIUM CONCENTRATION: [H2] = x = 0.00691 M 11. H2 (g) + CO2 (g) <====> CO (g) + H2O (g) If 1.50 mol of each chemical species are placed in a 3.00 L flask and allowed to achieve the equilibrium above, what mass of CO will be present at equilibrium ? (Keq = 3.59) [H2] = 1.50mol/3.00L = 0.500M [CO2] = 1.50mol/3.00L = 0.500M [CO2] = 1.50mol/3.00L = 0.500M [H2O] = 1.50mol/3.00L = 0.500M Q= [𝐶𝑂][𝐻2𝑂] 2 = [𝐻2][𝐶𝑂2] (0.500) 2 (0.500) = 1.00 Q = 1.00 < Keq = 3.59 Therefore, reaction will shift to the product side to increase Q value to that of Keq. H2 (g) + CO2 (g) ⇌ CO (g) H2O (g) INITIAL 0.500 0.500 0.500 0.500 CHANGE -x -x +x +x 0.500 - x 0.500 - x 0.500 + x 0.500 + x EQUIL. Keq = 3.59 = 3. 59 = 2 (0.500 + 𝑥) 2 (0.500 − 𝑥) (0.500 + 𝑥) (0.500 − 𝑥) 3. 59(0.500 - x) = 0.500 + x 3. 59(0.500) - 3. 59x = 0.500 + x 3. 59(0.500) - 0.500 = (1 + 3. 59)x ( 3.59(0.500) − 0.500) (1+ 3.59) =x x = 0.1545445822 [CO] = 0.500 + x = 0.500 + 0.1545445822 = 0.6545445822 M ( + 0.6545445822𝑚𝑜𝑙 𝐶𝑂 1𝐿 )(3. 00𝐿)( 28.01𝑔 𝐶𝑂 1𝑚𝑜𝑙 𝐶𝑂 ) = 55.0g CO