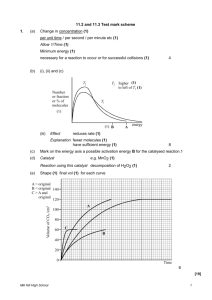
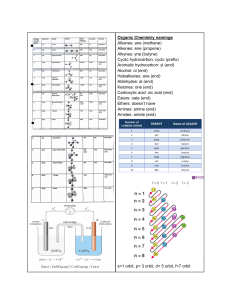
Interpreting Graphs Worksheet a. Write a balanced equation to represent the reaction studied. When the temperature is decreased, according to Le Chatelier's Principle (and the graph), the exothermic reaction (forward) is favoured. The system adjusts to replace lost heat, resulting in an increased product concentration and a decreased reactant concentration, and an increased K value because the forward reaction is favoured. b. How much time was required for the system to reach equilibrium? c. Calculate the approximate value for the equilibrium constant, Keq, using the concentrations at 60 s. d. Explain the changes 70 s after the initiation of the reaction. e. What changes in conditions might have been imposed on the system 120 s after the initiation of the reaction? f. Are any events taking place at 320 s? g. What differences would you have noticed if a catalyst had been present during the reaction?