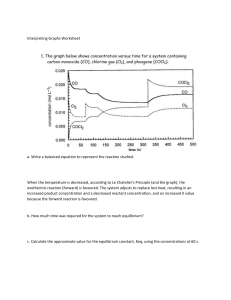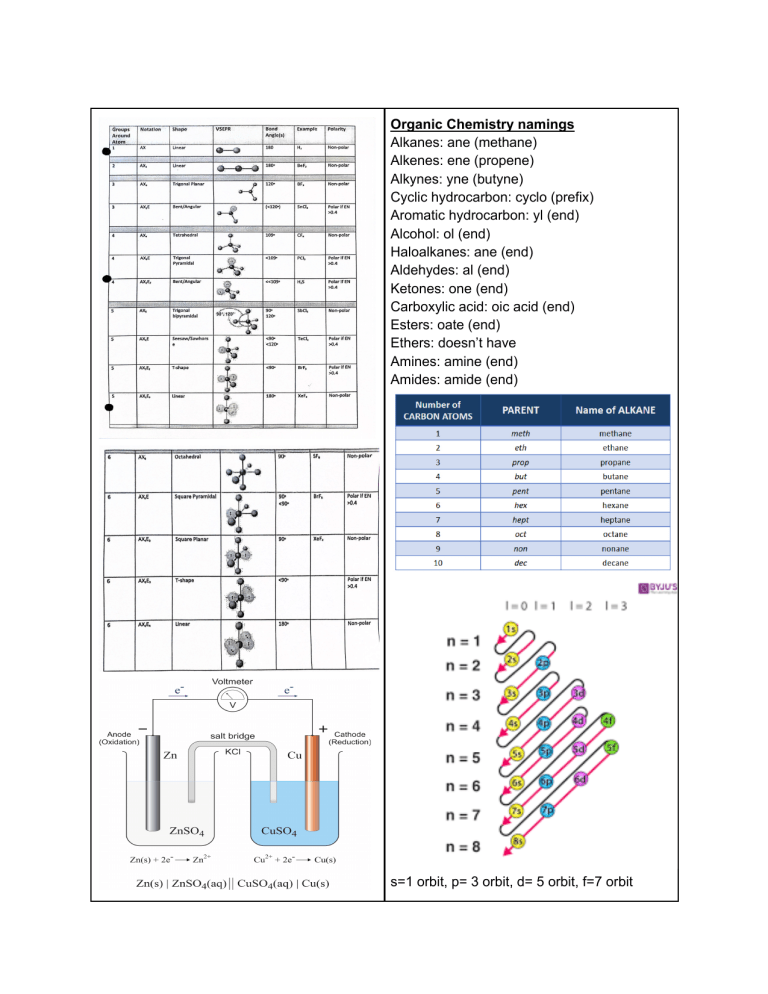
Organic Chemistry namings Alkanes: ane (methane) Alkenes: ene (propene) Alkynes: yne (butyne) Cyclic hydrocarbon: cyclo (prefix) Aromatic hydrocarbon: yl (end) Alcohol: ol (end) Haloalkanes: ane (end) Aldehydes: al (end) Ketones: one (end) Carboxylic acid: oic acid (end) Esters: oate (end) Ethers: doesn’t have Amines: amine (end) Amides: amide (end) s=1 orbit, p= 3 orbit, d= 5 orbit, f=7 orbit Rate of reaction: r = k(A)(B) Products -----------Reactants Hund’s law: says that when placing electrons in orbitals of equal energy, place one in each orbital before doubling up in order to arrive at the lowest energy configuration. Use ice chart to solve for esp from initial concentration to equilibrium Endothermic reactions have positive ΔH and gain heat (for example, ice melting) Aufbau’s law: states that energy levels must be filled from the lowest to the highest and you may not move on to the next level unless the previous level is full. Use the periodic table as a guide (read left to right): Exothermic reaction have negative ΔH and release heat Pauli law: when electrons do share an orbital, they must be of different “spin.” Q=MCT (m=mass) (c= specific heat capacity in J/mol) (t=temperature) Q can also = ΔH AROC: slope of secant = to average rate IROC: slope of tangent = instantaneous rate Keq>1: at equilibrium there is more product than reactant. Reaction is product favoured Keq=1: at equilibrium there is equal amount of product and reactants Keq<1: at equilibrium there are more reactants than products. Reaction is reactant favoured pH + pOH = 14 Arrhenius acid: produces hydronium ion in water Arrhenius base: produces hydroxide ion in water Bronsted-Lowry acid: Donates proton (H+) Bronsted-Lowry base: accepts proton Lewis acid: accepts electrons Lewis base: donates electrons Strong acids: HCl, Hbr, HI, HNO3, HClO3 Strong base: NaOh,KOH, Ca(OH)2,Ba(OH)2