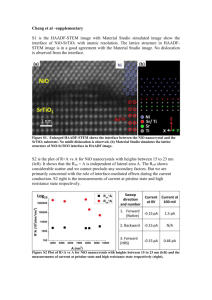
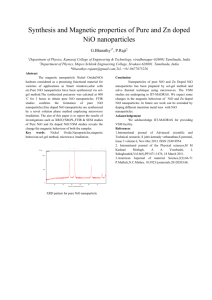
Dr. Babasaheb Ambedkar Marathwada University-431004 Chhatrapati Sambhajinagar, Maharashtra Department of Physics FACULTY OF SCIENCE AND TECHNOLOGY Project report on “Impact of fe2O3 Based Cr Doped NiO Nanocomposite for Supercapacitor Applications” Submitted by Munde Sushant Shivajirao (MSc IInd Year) Under The Guidance Dr. B.N. Dole Head and Professor Advanced Material Research Laboratory , Department Of Physics, Dr. BAMU Chhatrapati Sambhajinagar. Outline Of Presentation Objectives Introduction Literature Review Synthesis technique Experimental details Results and Discussion Conclusion References Objectives: To synthesize the Cr doped NiO nanocomposite by Co-precipitation method. Using different characterization techniques study the properties of the materials. To synthesize Cr doped NiO nanocomposite for super capacitor applications To examine the structures characterized by XRD INTRODUCTION Fe2O3( Iron Oxide): Molecular Weight :- 159.68 g/mol It is Ferromagnetic Molar Mass : 231.53 g/mol Density : 5 gm/cm3 Melting point : 15970 c ( 2907 F, 1870 K ) Boiling Point : 26230 c (2896 K, 4753 K ) CHROMIUM : Cr : Atomic No. :-24 1st element in a group It is steely grey, lustrous hard and brittle transition metal Atomic Weight :- 51.9961(+ or-)0.0006 Melting Point :- 2180K (19070c, 4840 F) Boiling Point :- 2944K ( 26710 c, 4840 F) Molar Heat capacity : 23.35 J/mol -K Nickel Oxide: NiO : Molar Mass :- 74.6928g/mol Melting point :- 15990 c ( 35510 F, 2228 K) It is a basic metal oxide. It is intrinsic P-type semiconductor with a wide band gap and good thermal and chemical stable Lightweight structural components for the aerospace industry As a material for temperature and gas sensors Nanowires and nanoalloys Literature review The author Ahmed, R., & Nabi synthesised that chromium doped NiO nano rods by using a facile hydrothermal method . The Cr0.06NiO has shown excellent capacitive performance as compared to other samples . This sample is consisted on Nano rods having large surface to volume ratio which is beneficial for redox reactions . XRD analysis has shown the crystalline nature of samples. The author Khan, A., Shkir, M., Ansari, S. A., Parveen, N., AlFaify, S., El-Toni, A. M., … Adil, S. F. report on the synthesis of Fe–NiO nanoparticles (Fe–NiO NPs) with a spherical shape via facile flash combustion synthesis. A structural test shows cubic formations of Fe–NiO NPs in Fe concentrations. The results show that the NiO doped with 5% of Fe exhibited superior capacitance performance (F4, 297.67 Fg1) compared to the other samples. SYNTHESIS TECHNIQUE: CO-PRECIPITATION METHOD: Coprecipitation as a simple technique is usually used for the preparation of inorganic- and metal-based nanoparticles. Iron oxide nanoparticles are widely famous in the biomedical field for their several advantages, and they are frequently synthesized using Coprecipitation method. This method has several advantages such as high product yield, environmentally friendly solvent, and narrow size distribution. Generally, the species forming the particles are dissolved in an aqueous medium(in the case of iron oxide nanoparticles, the species are FeCl3 ∙ 6H2O and FeCl2 ∙ 4H2O). The precipitation is induced by the addition of a base under stirring at room temperature under a nonoxidation atmosphere, leading to the formation of nanoparticles. The process variables that can govern the colloidal properties of the final particles are the type and ratio of salt used, reaction temperature, pH, and the ionic strength. Experimental Details: A] Synthesis of pure NiO : For the preparation of pure NiO 6.221 gm of Nickel Acetate, 3.5046 gm of HMTA were taken and to dissolve them 25 ml of DI water was seperately added in them . Both the materials were mixed and stirred for ½ hr . Drop by drop Ammonia was added in it for maintaining the Ph at 10.The mixture was then kept for heating about 2 hr . After heating the mixture was kept for coooling. After cooling the solution was filtered using filter paper. The material filtered was pure NiO. FLOW CHART 6.221gm of Nickel Acetate and 3.5046 gm HMTA disssolved separately in 25 ml DI water Stirred for ½ hr Ammonia was added dropwise for maintaining pH at 10 Solution was heated at 1000 c for 2 hrs. solution was kept for cooling and then for further filteration some amount of DI water was used After cooling the solution was filtered using the filter paper pure NiO was formed B] Synthesis of pure NiO with base Fe2O3 For the synthesis of pure NiO with Base Fe2O3, 6.221 gm of nickel acetate, 3.5046 gm of HMTA and 0.5 gm of Fe2O3 was taken. 25 ml of DI water was added in them separately and then it was kept in a beaker and then it was stirred for ½ hr. drop by drop Ammonia was added in it for maintaining Ph at 10 respectively. Then the solution was kept for heating at 1000 C for 2 hrs. After heating the solution was kept for cooling. After that the solution was given the filteration of DI water FOR removing the impurities. The solution was filtered using the filter paper. FLOW CHART 6.221 gm Nickel acetate, 3.5046 gm HMTA and 0.5 gm Fe2O3 were dissolved in 25 ml DI water seperately stirred for 1/2 hr Drop by Drop Ammonia added in it for pH at 10 solution was heated at 1000c for 2 hrs After heating solution was kept for cooling The solution was then filtered using filter paper and adding some amount of DI water for removing impurities Pure NiO with base Fe2O3 was formed C] Synthesis of Fe2O3 based Cr doped NiO For the synthesis of Fe2O3 based 5% Cr doped NiO ,6.221 gm of Nickel acetate, 3.5046 gm HMTA, 0.5 gm of Fe2O3 and 0.3330625 gm Chromium chloride was taken and 25 ml of DI water was added in them separately. Then the solution was stirred for ½ hr and drop by drop ammonia was added in it for maintaining Ph AT 10 respectively. The solution was then kept for heating at 1000 C for 2 hrs. After that the solution was kept for cooling. After cooling the solution was filtered using filter paper and adding some DI water in it for removing impurities. FLOW CHART 6.221 gm Nickel acetate, 3.5046 gm HMTA , 0.5 gm Fe2O3 ,0.3330625 gm chromium chloride were dissolved in 25 ml DI water seperately stirred for 1/2 hr drop by drop ammonia added for pH at 10 Solution was heated at 1000 C for 2 hrs After heating solution was kept for cooling The solutiom was then filtered using filter paper and adding some amount of DI water for removing impurities Fe2O3 based 5% Cr doped NiO was formed Results and Discussion Sample Crystalline Size Volume Dislocation Density Micro Strain Stacking fault Pure NiO 8.90039 25.77695 0.01232356 0.0035377 0.007418377 Fe2O3/Pure NiO 1.87844 37.23628 0.002834033 0.0018167 0.004889524 Fe2O3//5% Cr-NiO 7.99736 37.23628 0.015635317 0.2154823 0.005838419 (Fig.2: XRD results of pure NiO, Fe2O3 based NiO, Fe2O3 based Cr doped NiO) References: Ahmed, R., & Nabi, G. (2021). Enhanced Electrochemical Performance of Cr-doped NiO Nanorods for Supercapacitor Application. Journal of Energy Storage Khan, A., Shkir, M., Ansari, S. A., Parveen, N., AlFaify, S., El-Toni, A. M., … Adil, S. F. (2020). Onepot flash combustion synthesis of Fe@NiO nanocomposites for supercapacitor applications. Ceramics International. Rajan Lakra, Rahul Kumar, Parasanta Kumar Sahoo,Sandeep Kumar and Ankur Soam (Applications of Iron Oxide in supercapacitor) Conclusion The crystalline size of Pure NiO , Fe2O3 /NiO , Fe2O3 based Cr doped NiO was synthesised using Coprecipitation method The samples were characterized using XRD characterizaation and the functional group of material was found using FTIR . The samples were sent for futher tests like FESEM,,RAMAN UV-Vis spectra