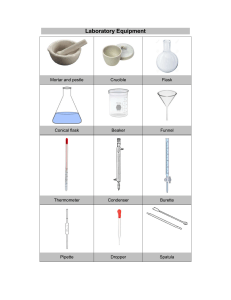
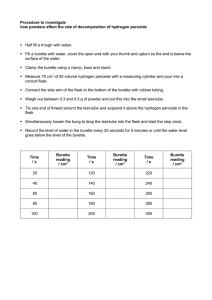
Chem - ATP Notes Look for the number of marks before answering the question Sometimes a straightforward question might expect more than one point to be made Be specific with volumes and solution names (name acids and alkalis used) Even if you can make a generalised statement Cracking of Long Chain alkanes Alkane and Catalyst → Heated Initially → Air passes through More accurate Burette → Variable volumes Volumetric pipettes → Measure fixed volume Lesser data → Poorer graph (2 marks) pH 1 - 3 Slow to add → Reaction begins while adding When larger beaker is used → Printed text - visible faster - less depth Repeat and compare Adv → More accurate; Dis.adv. → Takes longer/ slower Hydrated substance ↑ OBSERVATION → not "soluble in excess" Speak about heat → Speak about temperature Usage of polystyrene cup Whenever dissolving is concerned Air hole fully open Compare → Quantitative description In which solvent is solute more solvent in? Rate of rxn. → Mass loss Error → Tubing from flask should go into Con. Sulfuric Acid and tubing to gas jar → Not in acid General Flame test → CO → Limewater → Milky Residue → Wash and dry 2 Heating hydrated Chromium (III) Nitrate → Gives off oxygen Adding in excess → Can be test Disadvantage of burette → Slow/ takes more time/ can overshoot the required volume Experiment Design Reactions → State in which container → Beaker/ flask/ test-tube Control variables (How would you make it a fair test) → AT LEAST 3 Dependent variable → How would you measure STIR AND MIX Read question → Check what the question asks Jabez notes