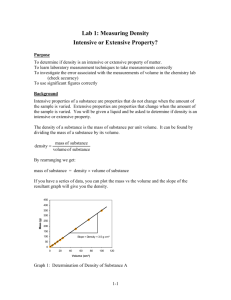
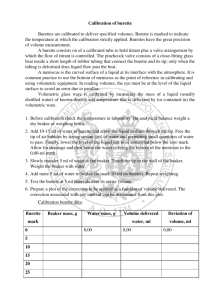
Calibration of Glassware: Glassware is commonly calibrated using a liquid of known, specific density, and an analytical balance. The procedure is to determine the mass of liquid the glassware will hold, and to divide this mass of liquid by the density of the liquid, obtaining the corresponding volume of liquid. Density is affected by temperature, so it is necessary to measure the liquid temperature and look up appropriate density values. Types of Glassware Laboratory glassware is made from borosilicate glassware which is resistant to thermal shock and chemical attack. Based on calibration, these glassware can be divided into two types; Type A Type A glassware is used for work requiring highest accuracy of results. The glassware bears the letter A and is calibrated to half the tolerance level of Class B glassware. It come with a calibration certificate bearing a unique serial number for establishing traceability. Type A glassware does not require further calibration. Type B Double the tolerance limit of class A glassware. It is used for routine work and requires calibration before putting to use. Before we move on to calibrating the glassware, we need to know how to calibrate an analytical balance which eases subsequent calibration of desired glassware with accurate results. Analytical Weighing Balance Calibration: The calibration method varies from balance to balance, yet we can use the following generalized method to calibrate an analytical weighing balance; 1. If the analytical balance is free from any physical damage, start by cleaning the weighing pan and other components using a lint-free cloth and a mild cleaning solution. 2. Power on the analytical balance and use the leveling bubble or leveling indicator to ensure that the balance is perfectly level. Adjust the balance feet as needed and zero the scale (tare) with nothing on the weighing pan. 3. Obtain calibrated weights suitable for the analytical scale's capacity, ensuring they are traceable to a national or international standard. 4. Now place the calibrated weights on the scale and adjust the readings to match the known weight. If there is a considerable mismatch, adjust the leveling feet until it matches the weight of the used calibrated weight. Volumetric Flasks: 1. Clean and rinse 3 volumetric flasks of desired size. Rinse the flasks 2 or 3 times with small (1-2 mL) portions of acetone making sure that you cover all inside surfaces with the acetone rinses, and discard these rinses. Allow the residual acetone to evaporate. Clearly label each flask, using the white space on the flask and a pencil. 2. Calibrate and level an analytical balance and then tare the balance. Determine the mass of each flask and its stopper, and record these masses. Make sure that you tare the balance before massing each flask. 3. Dispense a suitable volume of deionized water into a beaker, and measure and record the temperature of the deionized water. Use the table in your textbook and the water temperature to determine the correct density of water. Fill each of the flasks with deionized water to the calibration mark as shown below; 4. Return the deionized water to your beaker. Rinse the flasks 2 or 3 times with acetone as described in step 1. When the residual acetone has evaporated, repeat steps 2 and 3 until you have made triplicate measurements of the mass of water contained by each of the three flasks. 5. Calculate the corresponding volumes of water contained by each of the flasks, and calculate the mean and sample deviation for each flask. Pipettes: 1. Clean and rinse 3 pipettes of desired volume with distilled water. Then label the pipettes. 2. Measure the mass of a suitably sized beaker (for example a 50ml beaker) on the analytical balance, and record the mass. 3. Rinse the first pipette 3 times with deionized water by filling the pipette to the upper calibration mark, and discarding the rinse. 4. Finally, fill the pipette to the upper calibration mark, wipe excess water from the outside of the pipette with a lint free cloth or wipe and dispense this liquid into the beaker. 5. Measure and record the mass of beaker and water, discard the water, and dry the beaker. Repeat this procedure until you have triplicate measurements made for each pipette. 6. The mass of water dispensed from the pipette is found by subtracting the empty weight of the beaker from the weight of the beaker and water. 7. Calculate the corresponding volume of water delivered by each pipette, and calculate the mean and sample deviation for each pipette. Burette: 1. Select a suitably sized burette and carefully clean and rinse the burette with deionized water. 2. Determine the empty mass of a suitably sized beaker on the analytical balance. Record this mass. 3. Obtain a volume of deionized water (100 - 200 ml), and measure the temperature of the water. Fill the burette with deionized water to the 0 ml line. 4. Dispense a portion (20%) of deionized water from the burette into the beaker. For example; 5 ml for a 25 ml burette, or 10 ml for a 50 ml burette. Record the indicated volume from the burette. 5. Determine the mass of water dispensed using the analytical balance. 6. Repeat steps 4 and 5 until the volume of water in burette reaches the lowest mark (for example; 25 ml or 50ml). For each total volume of water dispensed, record the total mass. 7. Now you can discard the water in the beaker. Refill the burette to the 0 ml mark, and repeat the procedure (Steps 2 through 6) two more times, for a total of 3 calibrations. During each calibration, dispense different volumes of water into beaker (referring to step 4). 8. For each set of calibration data, determine the volume of water dispensed by dividing the total mass of water dispensed by the density of water at that temperature. Determine the difference in volumes by subtracting the calculated volume of water from the indicated volume of water. The differences can be either positive or negative, which are of significance. 10. Prepare a single calibration chart by plotting the differences in volume (yaxis), versus the indicated volume (x-axis). An example; Why do we use the calibration curve post-calibrating a burette? The burette used to acquire the above data doesn’t really deliver the exact volume that we read from the burette. The true volume delivered (calculated from the mass) is consistently less than it should be. However, it is not convenient to always weigh out whatever analyte is being delivered from the burette. Therefore, we use the calibration curve to correct the volumes delivered from the burette. References: 1. https://chem.libretexts.org/Courses/University_of_California_Davis/Chem_4A_Lab%3A_General_C hemistry_for_Majors_I/Chem_4A%3A_Laboratory_Manual/04_1%3A_Calibration_of_Volumetric_Gla ssware_(Experiment) 2. https://lab-training.com/volumetric-apparatus-use-calibration/ 3. https://www.mrclab.com/how-to-properly-calibrate-an-analytical-balance-for-accuratemeasurements 4. https://www.inscale-scales.co.uk/blogs/weighing-scales-blog/how-to-calibrate-an-analyticalbalance#:~:text=What%20does%20it%20mean%20to,anaytical%20balance%20with%20external%20ca libration. 5. https://uopachem.wordpress.com/2013/09/11/recap-calibrating-volumetric-glassware/ 6. ChatGPT (some definitions).