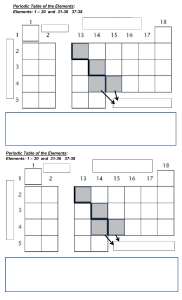
UNIT 1 ASSESSMENT- Periodic Trends Pre-activity thoughts 1. What do you think happens to the size of atoms of elements as you go down a column in the periodic table? If I had to make an uneducated guess about the trends on the size of an atom as the elements proceed down the column in the periodic table, My prediction would be that the size of an atom would increase. 2. Why did you predict this? I feel that the size of an atom would increase because the atomic radius of an atom should increase as the number of shells increases. 3. What do you think happens to the size as you go across a row on the periodic table from left to right? If I had to make an uneducated guess about the trends on the size of an atom as the elements proceed across the rows in the periodic table, My prediction would be that the size of an atom should stay the same, or at least extremely similar. 4. Why did you predict this? I feel that the size of an atom would increase because the atomic radius of an atom should not vary only if the proton is added to it. Additional Questions: Atomic Radii: 1. Explain the trends seen in atomic radii on the periodic table.[T/C] Typically, atomic radius shrinks over time (left to right). There are a few exceptions to this rule, but for the most part, it is consistent. At the same time as electrons are gradually added to the primary energy level, protons are gradually added to the nucleus. These electrons eventually gravitate toward the nucleus due to its increased positive charge. As the force of attraction between nuclei and electrons increases, the size of the atoms decreases. As one moves further to the right in a period, the impact becomes less noticeable because of the electron repulsions that prevent the atom from growing in size. Atoms often have an increasing atomic radius within a group (top to bottom). When the atomic number inside a group falls, the positive nuclear charge increases. However, the percentage of main energy levels that are occupied is also increasing. In comparison to orbitals with lower principal energies, those with higher principal energies have larger diameters. A gain in nuclear charge is overridden by the effects of having more fundamental energy levels, which causes an increase in atomic radius down a group. 2. Is there a general trend in atomic radii going diagonally down (or up) the periodic table? [A] In chemistry, some pairs of diagonally adjacent elements from the second and third periods of the periodic table (the first 20 elements) are said to exhibit a diagonal trend. The elements lithium (Li), magnesium (Mg), beryllium (Be), aluminium (Al), boron (B), silicon (Si), and others all have properties in common. For instance, the semiconductor properties of silicon and boron result in the formation of halides that hydrolyze in water and produce acidic oxides. The arrangement of elements in horizontal rows and vertical columns in the periodic table makes some connections more visible. 3. The values listed in the table given to you are for radius, but were drawn as the diameter. Does this invalidate the observations? [T] The actual size of the atoms in cm will be the double of the shown diagrams as the radius is half of diameter, so it can be said that the size will simply be double of the figure drown. Ionization Energy: 1. Explain the trends seen in ionisation energy on the periodic table. [T/C] The ionisation energy grows from left to right during the course of a period and drops from top to bottom in groups. As orbitals are added to a group from top to bottom, they correspond to larger values of the primary quantum number (n), which are often further from the nucleus. The initial ionisation energy is lower because the outermost electrons are further from the nucleus, where they are less strongly attracted to the nucleus and simpler to expel. 2. Is there a general trend in ionisation energy going diagonally down (or up) the periodic table? [A] Ionisation energies rise diagonally from the lower left to the top right of the periodic table. Minor exceptions from this trend can be accounted for by stable electronic configurations, often known as pseudo noble gas configurations, in either the parent atom or the ion that results from them. 3. Account for any anomalies you may observe in trends. [T] Usually the trend shows the energy increases from left to right however for elements Aluminium(Al), Sulphur (S) Gallium(Ga), Selenium(Se). These elements have a smaller energy than the element to the element to their left, which is an abnormality. Ionic Radii: 1. Explain the trends seen in ionic radii on the periodic table. [T/C] The ionic radius of elements increases along a group in the periodic table when atoms add an additional shell (number of electrons) to their structure. As you progress from left to right through the periodic table, the ionic radius decreases. 2. Why are the values for noble gases not listed? [A] The ionic radius does not exist for the noble gases. The reason for that is that because ions are charged particles. Atoms form charged particles known as ions when they absorb or lose electrons. However this is not the case for noble gases. So we can observe the missing values of ionic radius Electronegativity: 1. Explain the trends seen in electronegativity on the periodic table. [T/C] Electronegativity on the periodic table normally increases from left to right across a period and decreases as you move down a group. The most electronegative elements are thus found in the top right corner of the periodic table, whereas the least electronegative ones are found in the bottom left. 2. Why would noble gases have no electronegativity values listed? [A] Inert gases have no inclination to attract the shared electrons, hence their electronegativity is 0. Since the noble gases already have eight electrons in their outer shells, they don't need to attract any more.