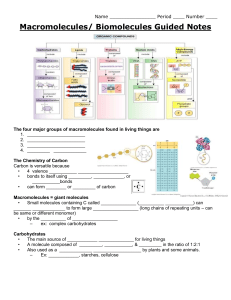
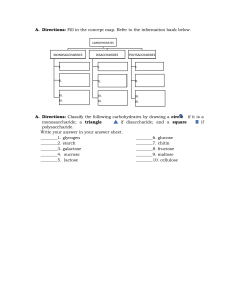
BIOLOGICAL MOLECULES 4 BIOMOLECULES UNFOLDING THE STRUCTURES AND FUNCTIONS OF BIOLOGICAL MOLECULES AFTER GOING THROUGH THIS LESSON, YOU ARE EXPECTED TO: • DEFINE BIOLOGICAL MOLECULES; • IDENTIFY THE ROLE OF EACH BIOLOGICAL MOLECULE IN SPECIFIC METABOLIC PROCESSES; • DIFFERENTIATE EACH BIOLOGICAL MOLECULES ACCORDING TO THEIR STRUCTURE AND FUNCTION BIOLOGICAL MACROMOLECULES •THERE ARE FOUR MAJOR CLASSES OF BIOLOGICAL MACROMOLECULES (CARBOHYDRATES, LIPIDS, PROTEINS AND NUCLEIC ACIDS) • THEY ARE ORGANIC, MEANING THEY CONTAIN CARBON. IN ADDITION, THEY MAY CONTAIN HYDROGEN, OXYGEN, NITROGEN AND ADDITIONAL MINOR ELEMENTS BIOLOGICAL MACROMOLECULES MACROMOLECULES ARE POLYMERS, BUILT FROM MONOMERS. • MACROMOLECULES ARE FORMED BY SHARING ELECTRONS AND FORM COVALENT BONDS. • IT IS FACILITATED BY THE FOLLOWING PROCESSES A. DEHYDRATION SYNTHESIS B. HYDROLYSIS DEHYDRATION SYNTHESIS • ALSO CALLED CONDENSATION REACTION • REACTION THAT OCCURS TO BUILD POLYMERS • WATER MOLECULE IS LOST AS A BY-PRODUCT • REQUIRES ENERGY INPUT, ENZYME. HYDROLYSIS • POLYMERS ARE BROKEN DOWN INTO MONOMERS • MEANS “TO SPLIT WATER” • A REACTION IN WHICH A WATER MOLECULE IS USED DURING THE BREAKDOWN. • RELEASES ENERGY • ACCELERATED BY ENZYME ENZYME AN ORGANIC MOLECULE THAT SPEEDS UP A CHEMICAL REACTION BY LOWERING THE AMOUNT OF ENERGY REQUIRED TO START A CHEMICAL REACTION Components in Enzyme Activity: • Substrate • Active site • Enzyme • Product Factors that affect rate of enzyme activity: • pH level • temperature Activity Time! EFFECTS OF pH AND TEMPERATURE ON ENZYME ACTION Using the data in the chart, plot a graph showing the rate of enzyme action for the enzymes Pepsin and Trypsin at varying pH. pH Rate of Pepsin Action Rate of Trypsin Action 1 1 0 2 3 0 3 7 0 4 3 0 5 1 1 6 0 3 7 0 7 8 0 9 9 0 7 10 0 3 11 0 1 1 12 0 0 0 13 0 0 14 0 0 pH-Enzyme Activity Rate 10 9 R a t e 8 7 6 5 4 3 2 1 2 3 4 5 6 7 8 9 10 pH 11 12 13 14 Using the data in the chart, plot a graph showing the rate of enzyme action at different temperature. Temp 0C Reaction rate 10 1.0 15 1.5 20 2.5 25 3.5 30 5.5 35 7.5 40 9.5 45 8.5 50 7.0 55 4.0 60 0.0 Temperature-Enzyme Activity Rate R a t e 10 9.5 9 8.5 8 7.5 7 6.5 6 5.5 5 4.5 4 3.5 3 2.5 2 1.5 1 5 10 15 20 25 30 35 40 45 50 55 60 65 70 Temperature Analysis: 1. At what pH is trypsin most effective? How about pepsin? 2. What is the best temperature for this enzyme to work? CARBOHYDRATES • THEY ARE ORGANIC COMPOUNDS MADE UP OF CARBON, HYDROGEN AND OXYGEN. • CARBOHYDRATES CAN BE REPRESENTED BY THE STOICHIOMETRIC FORMULA (CH2O). • CARBOHYDRATES INCLUDE SUGARS AND THE POLYMERS OF SUGARS • THE SIMPLEST CARBOHYDRATES ARE MONOSACCHARIDES, OR SIMPLE SUGARS • CARBOHYDRATE MACROMOLECULES ARE POLYSACCHARIDES, POLYMERS COMPOSED OF MANY SUGAR BUILDING BLOCKS CARBOHYDRATES • TYPES OF CARBOHYDRATES 1. MONOSACCHARIDES 2. OLIGOSACCHARIDES 3. POLYSACCHARIDES MONOSACCHARIDES • Monosaccharides (mono- = “one”; sacchar- = “sweet”) are simple sugars • Most monosaccharide names end with the suffix -ose. If the sugar has an aldehyde group (the functional group with the structure R-CHO), it is an ALDOSE, and if it has a ketone group (the functional group with the structure RC(=O)R'), it is a KETOSE. • Depending on the number of carbons in the sugar, they can be trioses (three carbons), pentoses (five carbons), and/or hexoses (six carbons). ALDOSE KETOSE 6 CARBONS 6 CARBONS - OSE - OSE ALDOHEXOSE KETOHEXOSE MONOSACCHARIDES • GLUCOSE (C6H12O6) IS THE MOST COMMON MONOSACCHARIDE. EXAMPLES: • GLUCOSE – A 6 CARBON ALDOSE THAT IS THE PRODUCT OF PHOTOSYNTHESIS AND THE SUBSTRATE FOR RESPIRATION THAT PROVIDES ENERGY FOR CELLULAR ACTIVITIES. • FRUCTOSE – A CARBON KETOSE THAT IS FOUND IN MANY PLANTS AND IS OFTEN BONDED TO GLUCOSE. • GALACTOSE – A 5 CARBON ALDOSE THAT FORMS PART OF THE NUCLEIC ACIDS. OLIGOSACCHARIDES • OLIGO MEANS FEW • CARBOHYDRATE CHAINS CONTAINING 2–10 SUGAR UNITS COVALENTLY BONDED BY A GLYCOSIDIC LINKAGE. • FUNCTIONS AS ENERGY SOURCE, SWEETENER AND DIETARY COMPONENT. • TYPES: DISSACCHARIDES- 2 UNITS OF SUGAR TRISACCHARIDES- 3 UNITS OF SUGAR TETRASACCHARIDES- 4 UNITS OF SUGAR… OLIGOSACCHARIDES EXAMPLES: • MALTOSE (GLUCOSE + GLUCOSE) – MALT SUGAR OFTEN FOUND IN SPROUTING GRAINS, MALT-BASED ENERGY DRINKS OR BEER. • SUCROSE (GLUCOSE + FRUCTOSE) – FOUND IN TABLE SUGAR PROCESSED FROM SUGAR CANE, SWEET FRUITS AND STORAGE ROOT LIKE CARROTS. • LACTOSE (GLUCOSE + GALACTOSE) – MILK SUGAR THAT IS A SOURCE OF ENERGY FOR INFANTS; AN ENZYME CALLED LACTASE IS REQUIRED TO DIGEST IT. POLYSACCHARIDES EXAMPLES: STORAGE POLYSACCHARIDES ARE LARGE MOLECULES RETAINED IN THE CELL AND ARE INSOLUBLE IN WATER. 1. STARCH – AMYLASE IS UNBRANCHED STARCH FORMING A HELICAL STRUCTURE WHILE AMYLOPECTIN IS BRANCHED STARCH, THESE ARE PRESENT IN PLANTS PARTS LIKE POTATO TUBERS, CORN AND RICE AND SERVE AS MAJOR SOURCES OF ENERGY. 2. GLYCOGEN – FOUND IN ANIMALS AND FUNGI; OFTEN FOUND IN LIVER CELLS AND MUSCLE CELLS. POLYSACCHARIDES STRUCTURAL POLYSACCHARIDES 1. CELLULOSE – TOUGH SHEET LIKE STRUCTURES THAT MAKE UP PLANT AND ALGAL CELL WALLS THAT MAY BE PROCESSED TO FORM PAPER AND PAPER-BASED PRODUCTS. 2. CHITIN – USED FOR STRUCTURAL SUPPORT IN THE WALLS OF FUNGI AND IN EXTERNAL SKELETONS OF ARTHROPODS. 3. PEPTIDOGLYCAN – USED FOR STRUCTURAL SUPPORT IN BACTERIAL CELL WALLS. Review of Carbs 1. The process involved in building up glucose and fructose to form sucrose. 2. Monomer of carbohydrates found in animals. 3. A disaccharide form when 2 molecules of glucose undergo dehydration synthesis. 4. An organic compound composed of carbon, hydrogen and oxygen. 5. An enzyme necessary to break down lactose into glucose and galactose. 6. 2 Important polysaccharides found in plants and animals which function as storage. 7. --8. 2 important polysaccharides found in plants and bacteria that forms as structural components. 9. --10. Name the Monosaccharide 1. The process involved in building up glucose and fructose to form sucrose. DEHYDRATION SYNTHESIS 2. Monomer of carbohydrates found in animals. GALACTOSE 3. A disaccharide form when 2 molecules of glucose undergo dehydration synthesis. MALTOSE 4. An organic compound composed of carbon, hydrogen and oxygen. CARBOHYDRATES 5. An enzyme necessary to break down lactose into glucose and galactose. LACTASE 6. 2 Important polysaccharides found in plants and animals which function as storage. STARCH 7. --GLYCOGEN 8. 2 important polysaccharides found in plants and bacteria that forms as structural components. CELLULOSE 9. --PEPTIDOGLYCANS 10. Name the Monosaccharide ALDOTETROSE LIPIDS • LIPIDS ARE A CLASS OF LARGE BIOMOLECULES THAT ARE NOT FORMED THROUGH POLYMERIZATION. • THEY MAY HAVE SOME OXYGEN ATOMS IN THEIR STRUCTURE BUT THE BULK IS COMPOSED OF ABUNDANT NON-POLAR CARBON-HYDROGEN BONDS. • THEY PLAY IMPORTANT ROLES IN PLASMA MEMBRANE STRUCTURE AND SERVE AS PRECURSORS FOR IMPORTANT REPRODUCTIVE HORMONES. FATS (TRIACYLGLYCEROLS OR TRIGLYCERIDES) • ENERGY STORAGE, CUSHIONING OF VITAL ORGANS (ADIPOSE TISSUE) AND INSULATION • FATS ARE CONSTRUCTED FROM TWO TYPES OF SMALLER MOLECULES: GLYCEROL AND FATTY ACIDS • GLYCEROL IS A THREE-CARBON ALCOHOL WITH A HYDROXYL GROUP ATTACHED TO EACH CARBON • A FATTY ACID CONSISTS OF A CARBOXYL GROUP ATTACHED TO A LONG CARBON SKELETON FATS EXAMPLES: • SATURATED FAT – ANIMAL PRODUCTS SUCH AS BUTTER AND LARD HAVE A LOT OF SATURATED FATTY ACIDS. • UNSATURATED FATS – PLANT AND FISH OILS HAVE UNSATURATED FATTY ACIDS. • TRANS FAT – MAY BE PRODUCED ARTIFICIALLY THROUGH THE PROCESS OF HYDROGENATION. PHOSPHOLIPIDS • MAJOR COMPONENT OF CELL MEMBRANE MADE OF GLYCEROL, FATTYACIDS AND PHOSPATE GROUP. • PHOSPHATE GROUP IS HYDROPHILIC AND IS CALLED THE ‘HEAD’ OF THE MOLECULE. • FATTY ACIDS ARE HYDROPHOBIC AND FORM THE ‘TAILS’ OF THE MOLECULE. STEROIDS AND STEROLS • • • • REGULATE FLUIDITY OF CELL MEMBRANE. BASE OF SEX HORMONES. EMULSIFICATION OF FATS DURING DIGESTION. CHOLESTEROL FOUND IN CELL MEMBRANE REGULATES THE RIGIDITY OF THE CELL MEMBRANE AND ARE THE BASE MATERIAL FOR THE PRODUCTION OF SEX HORMONES LIKE ESTRADIOL AND PROGESTERONE. WAXES • SIMPLE LIPIDS CONSISTS OF LONG CHAIN ALCOHOL AND A FATTY ACID JOINED BY ESTHER. • THEY FOUND AS COATING OF STEM AND LEAVES TO PREVENT EXCESSIVE LOSS OF WATER IN PLANTS. • IN INSECTS, COVER BODY SURFACES TO RESTRICT MOVEMENT OF WATER ACROSS THE CUTICLE TO PREVENT THE BODY FROM DRYING. PROTEINS • PROTEINS ARE ONE OF THE MOST ABUNDANT ORGANIC MOLECULES IN LIVING SYSTEMS AND HAVE THE MOST DIVERSE RANGE OF FUNCTIONS OF ALL MACROMOLECULES. • THEY MAY BE STRUCTURAL, REGULATORY, CONTRACTILE OR PROTECTIVE; THEY MAY SERVE IN TRANSPORT, STORAGE OR MEMBRANES; THEY MAY BE TOXINS OR ENZYMES. • AMINO ACIDS ARE THE MONOMERS THAT MAKE UP PROTEINS. POLYPEPTIDES – polymer of protein TYPES AND FUNCTIONS OF PROTEINS NUCLEIC ACID • NATURALLY OCCURRING CHEMICAL COMPOUND THAT IS CAPABLE OF BEING BROKEN DOWN TO YIELD PHOSPHORIC ACID, SUGARS, AND A MIXTURE OF ORGANIC BASES (PURINES AND PYRIMIDINES). • NUCLEIC ACIDS ARE POLYNUCLEOTIDES, EACH NUCLEIC ACID CONTAINS FOUR OF FIVE POSSIBLE NITROGEN-CONTAINING BASES: ADENINE (A), GUANINE (G), CYTOSINE (C), THYMINE (T), URACIL (U). A. Identify each as Carbohydrates, protein or lipid. 1.Starch 2.Cholesterol 3.Steroid 4.Glycogen 5.Enzyme 6.Saturated fat 7.Polypeptide chain 8.Glucose 9. Polysaccharide 10. Phospholipid 11. Glycerol 12. Monosaccharide 13. Cellulose 14. Amino acid 15. Unsaturated fatty acid B. Match each molecule with its components. COLUMN A 1. Amino acid monomer 2.Fatty acids, carbon rings 3.Glucose, Fructose 4.Glucose monomers 5. Glycerol, fatty acids 6.Glycerol, fatty acids, phosphates 7. Nucleotide monomers 8. Sugar, phosphate and nitrogen bases COLUMN B a. b. c. d. e. f. g. h. i. Cellulose Fat Nucleotide Nucleic acid Phospholipid Protein Sucrose Wax Glucose A. Identify each as Carbohydrates, protein or lipid. 1.Starch 2.Cholesterol 3.Steroid 4.Glycogen 5.Enzyme 6.Saturated fat 7.Polypeptide chain 8.Glucose 9. Polysaccharide 10. Phospholipid 11. Glycerol 12. Monosaccharide 13. Cellulose 14. Amino acid 15. Unsaturated fatty acid B. Match each molecule with its components. COLUMN A 1. Amino acid 2.Fatty acids, carbon rings 3.Glucose, Fructose 4.Carbohydrates monomer 5. Glycerol, fatty acids 6.Glycerol, fatty acids, phosphates 7. Nucleic acid monomers 8. Sugar, phosphate and nitrogen bases COLUMN B a. b. c. d. e. f. g. h. i. Cellulose Fat Nucleotide Nucleic acid Phospholipid Protein monomer Sucrose Steroid Glucose