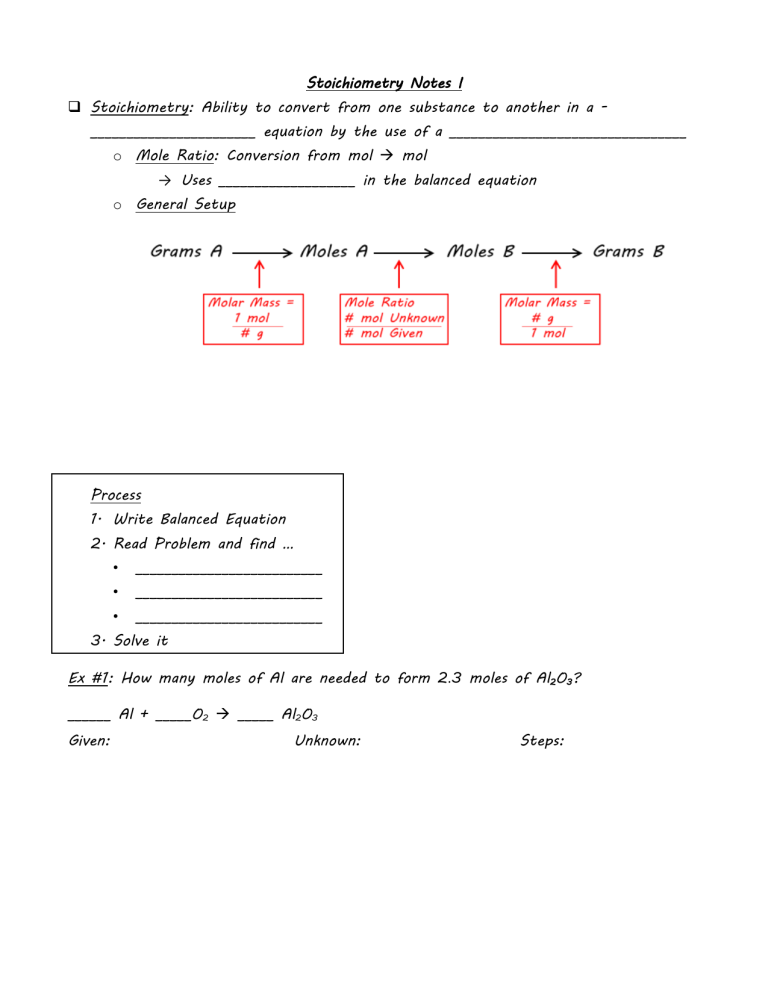
Stoichiometry Notes I Stoichiometry: Ability to convert from one substance to another in a _______________________ equation by the use of a _________________________________ o Mole Ratio: Conversion from mol mol → Uses ___________________ in the balanced equation o General Setup Process 1. Write Balanced Equation 2. Read Problem and find … • __________________________ • __________________________ • __________________________ 3. Solve it Ex #1: How many moles of Al are needed to form 2.3 moles of Al2O3? ______ Al + _____O2 _____ Al2O3 Given: Unknown: Steps: Ex #2: Calculate the mass of MgO produced from the reaction of 2.50 mol Mg and excess Oxygen. Given: Unknown: Steps: Ex #3: Calculate the # of moles of H2O produced when 8.08 g H2 reacts with an excess of O2. Given: Unknown: Steps: