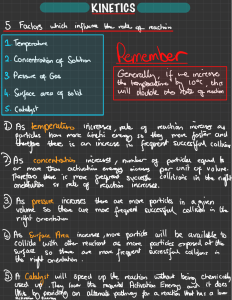
Topic 6: Chemical Kinetics 6.1 Collision Theory and Rates of Reaction ● What makes particles react? ● How can this be used to explain how we change the rate of a reaction? 1 2 of 39 © Boardworks Ltd 2007 What does rate of reaction mean? The speed of different chemical reactions varies hugely. Some reactions are very fast and others are very slow. The speed of a reaction is called the rate of the reaction. What is the rate of these reactions? rusting Very slow -------3 of 39 baking Slow ------ explosion Very fast ---------© Boardworks Ltd 2007 Why study rates? What is the real-world context? Time is money in industry, the faster the reaction can be done, the more economic it is. Which reactions do you know? Health and Safety Issues: The faster a reaction, the more likely there might be health and safety issues to contend with. The physical state or concentration of a material can be an important factor. Mixtures of flammable gases in air present an explosion hazard (gas reactions like this are amongst the fastest reactions known). If the flammable/explosive gas is in low concentration, there may be no risk, but you need to know the safe limits! e.g. Methane gas in mines, petrol vapour etc. are all potentially dangerous situations so knowledge of 'explosion/ignition threshold concentrations', ignition temperatures and activation energies are all important knowledge to help design systems of 4 operation to minimise risks. Collision Theory Reactions take place when particles collide with a certain amount of energy. The minimum amount of energy needed for the particles to react is called the activation energy, and is different for each reaction. The rate of a reaction depends on two things: ● the frequency of collisions between particles ● the energy with which particles collide. If particles collide with less energy than the activation energy, they will not react. The particles will just bounce off each other. 5 of 39 © Boardworks Ltd 2007 In this video learn about Collision Theory and find out what is necessary for reactions to take place. https://www.youtube.com/watch?v=eSInI1xHvh4 Am booklet page 76 Study very well !!!! For a reaction to take place; successful collisions must occur 1- Particles must collide 2- with sufficient energy 3- corect orientation It is the minimum amount of energy needed to start a reaction MCQ Am booklet Page 78 Reactants could be of different states and they can react! Page 83 Based on the definition of rate: Concentration unit is mol.dm-3 Time unit is seconds (s) Unit of rate= mol.dm-3 s Defining the Rate of Reaction The rate of reaction is expressed as the change in concentration of a particular reactant/product per unit time. Amount of products increasing with time Being produced. ◎ Where: ○ ∆[R] is change in concentration of reactants ○ ∆[P] is change in concentration of products ○ ∆t is change in time Amount of reactants are decreasing With time, getting used up 9 ◎ Official Units: mol dm-3 s-1 (‘moles per cubic decimetre per second’) Slower and slower! Reactions do not proceed at a steady rate. They start off at a certain speed, then get slower and slower until they stop. As the reaction progresses, the concentration of reactants decreases. This reduces the frequency of collisions between particles and so the reaction slows down. 0% initially High concentration of reactants. no products formed yet 10 of 25% 50% 75% As the reaction progresses, the amount of reactants decreases and amount of products increases reactants product 100% At the end, all reactants are Used up and maximum number of products are formed percentage completion of reaction © Boardworks Ltd 2007 Graphing rates of reaction With time the rate of reaction decreases with time, (Reaction slows) until it stopes. 11 of © Boardworks Ltd 2007 Reactant–product mix 12 of © Boardworks Ltd 2007 The reactant/product mix E A 13 of 39 D B C © Boardworks Ltd 2007 Am booklet page 93 Reaction stops when the the curve Becomes a horizontal (flat) line Maximum yield; maximum amount of products formed Graph line starts to flatten Slope gets less steep thus rate deceases Initillay the rate is high Steep slope Rate of reaction decreases with time, since the concentration of the reactants decreases; Thus less frequent collisions Refresher Am booklet pages 102-103 Change in the concentration of reactants or products with respect to time. Or write the equation. 1- particles must collides 2- with the sufficient energy 3- correct orientation Refresher Am booklet page 104 The minimum amount of energy needed to start a reaction. Instantaneous and Initial Rate of a reaction How to draw tangents to a curve ◎ Determining the rate at a particular point in time (instantaneous rate and initial rate) requires us to draw tangents to the graph line 17 You need to know how to get the rate of the reaction using the graph: When drawing the tangent to the curve at a specific point Be careful to hold the ruler in the correct side Touching the curve at only the specific point Am booklet page 106. Write and solve in the space provided Calculating rate - at various points in a reaction 1. Calculate the average rate for the reaction from start to end. Where is the end? The reaction ends here. (120, 58) Average rate = 58 - 0 = 0.483 cm3/s 120-0 Is the average rate for the reaction The reaction starts here (0,0) 19 The slope of the tangent to the line joining the starting point and the end point Am booklet page 106. Write and solve in the space provided Calculating rate - at various points in a reaction 2. Calculate the average rate for the first 50 seconds of each reaction. Average rate= 53 - 0 (50, 53) = 1.06 cm3/s 50 - 0 20 Slope of the line Is the average rate 0,0 Am booklet page 106. Write and solve in the space provided Calculating rate - at various points in a reaction 3. Calculate the average rate between 10 - 90 seconds in each reaction. (90, 57) Average rate = 57 - 18 90 - 10 = 0.4875 cm3/s 21 Slope of the line Is the average rate (10, 18) Am booklet page 106. Write and solve in the space provided Calculating rate - at various points in a reaction 4. Calculate the instantaneous rate at 25 seconds Instantaneous rate is the rate of the reaction at a specific instance in time Draw a tangent to the graph at the point. (Make sure the line touches the cur ve at only the specific point asked for. (25, 38) get the slope of the tangent. (It is recommended to extend the line until the y-axis), choose t wo points on the tangent and get the slope. 22 the slope of the tangent is the rate 0, 10 Slope = 38 - 10 = 1.12 cm3/s 25 - 0 Am booklet page 106. Write and solve in the space provided Calculating rate - at various points in a reaction 5. Calculate the initial rate of reaction (i.e. t = 0) Initial rate is the instantaneous rate at time t= 0 (Start) Draw a tangent to the cur ve and t=0 Calculate the slope of the tangent 37-0 = 17 cm3/s 20-0 23 (20, 37) (0,0) What happens to rate as the reaction proceeds? ◎ Look at the calculated rates for each stage of the previous reaction. What do you see? The highest rate was for the initial rate ◎ Why do you think this happens? Rate decreases, since the curve gets flattened (less steep) Anything? Wonder The rate decreases and slope of the tangents to the cur ve of increased Times decreases; since the concentration of the reactants are decreasing, less frequent collosions, thus reaction slows down with time until it stops when reactants are used up. 24 ◎ Analysis of graphical and numerical data from rate experiments. Average rate bet ween time t=0 and t= 20 Get the slope For this line 25 Calculation of reaction rates from tangents of graphs of concentration, volume or mass vs time should be covered. 26 Make sure you know how to get these from the graph!!!!! Review What is the best definition of rate of reaction? The time it takes to use up all the reactants The rate at which all the reactants are used up The time it takes for one of the reactants to be used up The increase in concentration of a product per unit time Increase or decrease is another word for a change in an amount The rate must be defined with respect to time!!!! 27 A. B. C. D. Am booklet page 74 HW decrease is the change in the concentration (or amount) of reactants or products per unit time. (Or write the mathematical equation) Rate of reaction decreases with time since the concentration of reactants is decreasing, less frequent collisions. initially, the rate is highest (fastest/ steep line) with a high concentration of reactants. With time the reactants are getting used up, thus slower reaction, the line gets less steep, until it flattens. flat line indicates rate is zero , where the reaction stops, reactants are used up. Changing the rate of reactions Anything that increases the number of successful collisions between reactant particles will speed up a reaction. What factors affect the rate of reactions? ● increased temperature ● increased concentration of dissolved reactants, and increased pressure of gaseous reactants ● increased surface area of solid reactants ● use of a catalyst. © Boardworks Ltd 2007 MUST BE STUDIED!! V. IMP For aqueous solutions Particle size Collisions and reactions: summary Complete the sentences about the factors affecting rate of reactions: 1. Changing the collisions. Temperature affects both the frequency and energy of Concentration 2. Changing the affects the frequency of collisions of dissolved reactants. (aqueous solutions) 3. Changing the solid 31 of 39 Particle size (surface area) affects the frequency of collisions involving a © Boardworks Ltd 2007 32 of 39 © Boardworks Ltd 2007 Effect of temperature on rate The higher the temperature, the faster the rate of a reaction. In many reactions, a rise in temperature of 10 °C causes the rate of reaction to approximately double. Why does increased temperature increase the rate of reaction? At a higher temperature, particles have more energy. This means they move faster and are more likely to collide with other particles. When the particles collide, they do so with more energy, and so the number of successful collisions increases. 33 of 39 © Boardworks Ltd 2007 Temperature and particle collisions Higher temperature will have more frequent collisions 34 of 39 © Boardworks Ltd 2007 Temperature and batteries Why are batteries more likely to rundown more quickly in cold weather? At low temperatures the reaction that generates the electric current proceeds more slowly than at higher temperatures. This means batteries are less likely to deliver enough current to meet demand. 35 of 39 © Boardworks Ltd 2007 36 of 39 © Boardworks Ltd 2007 Effect of concentration on rate of reaction The higher the concentration of a dissolved reactant, the faster the rate of a reaction. Why does increased concentration increase the rate of reaction? At a higher concentration, there are more particles in the same amount of space. This means that the particles are more likely to collide and therefore more likely to react. Shorter distance bet ween the particles ; Less time to collide lower concentration 37 of 39 higher concentration © Boardworks Ltd 2007 Concentration and particle collisions more particles in a specific volume. Particles travel less distance to collide. More frequent collisions; thus higher rate 38 of 39 © Boardworks Ltd 2007 The Maxwell-Boltzmann Energy Distribution Describes how energy is distributed in a collection of particles: You need to know how to: 1- Label the y and x axis. 2- label activation energy 3- draw the curve 4- label t wo areas under the curve green and purple) Number of particles That do not have enough energy to collide successfully. (these particles can not react) The curve must not touch the x-axis (this means there are no particles) Number to particles with enough energy to collide successfully and react. They have energy that is equal to or greater than the activation energy. Effect of Changing the temperature on Maxwell Boltzmann Watch the video on the powerpoint. Page 77 Area under the curve Shows the number of with insufficient energy to collide successfully and react Area under the curve Shows the number of particles with sufficient energy that is greater or equal to activation energy, and are able to collide successfully and react. E Ea Page 75 Increase in the temperature results in: 1. increase in the average kinetic energy of the particles 2. more particles have sufficient energy to react 3. more frequent collisions Area under the curve increases; More particles have sufficient energy (Ea> E) to react T T1 > T Ea Activation energy: This is the minimum amount of energy needed to start the reaction Energy Page 97 For higher temperature: Make sure that the peak is switched to the right, and flattens a bit. for colder temperature make sure that the peak is tending towards the y-axis and the peak is sharp Increase in the temperature results in: 1. increase in the average kinetic energy of the particles 2. more particles have sufficient energy to react (shaded area under the cur ve gets bigger) 3. more frequent collisions Repeated practice Make sure to draw in pencil and label the axis; activation energy, and the t wo curves Page 101 Effect of pressure on rate of reaction For reactants that are gasses ONLY Why does increasing the pressure of gaseous reactants increase the rate of reaction? As the pressure increases, the space in which the gas particles are moving becomes smaller. The gas particles become closer together, increasing the frequency of collisions. This means that the particles are more Note: Pressure is inversely proportional to volume; likely to react. Decreasing the Volume means increasing the pressure lower pressure 45 of 39 higher pressure © Boardworks Ltd 2007 46 of 39 © Boardworks Ltd 2007 Effect of surface area on rate of reaction OR particle size OR state of division for Solid reactants ONLY Any reaction involving a solid can only take place at the surface of the solid. If the solid is split into several pieces, the surface area increases. What effect will this have on rate of reaction? low surface area Larger particle size high surface area Smaller particle size This means that there is an increased area for the reactant particles to collide with. The smaller the pieces, the larger the surface area. This means more collisions and a greater chance of reaction. 47 of 39 © Boardworks Ltd 2007 Surface area and particle collisions Smaller surface area means less particles are exposed. Only the outer particles will react. 48 of 39 Greater surface area means more particles are exposed to the other reactants Thus more frequent collisions. © Boardworks Ltd 2007 Pages 74-75 State: Increasing the temperature; increases the rate of the reaction, faster reaction. Explain: increasing the temperature; increases the average KE of the particles; more particles will have enough energy to react; thus more frequent collisions State: Increasing the concentration of the aqueous solution; increases the rate of the reaction. Explain: more particles in a specific volume; less space bet ween them, thus more frequent collisions State: Increasing the particle size; means decreasing the surface area; thus the rate of the reaction decreases. Explain: decreasing the surface area, less particles are exposed the the other reactant, thus less frequent collisions Page 75 Changes that could increase the rate: 1- for SOLID Mg: increase the surface area; by using smaller pieces (or particle size) 2- for AQUEOUS Solution Hal: use a higher concentration 3- increase the temperature Changes that could decrease the rate: 1- for SOLID Mg: decrease the surface area; by using larger pieces (or particle size) 2- for AQUEOUS Solution Hal: use a lower concentration 3- decrease the temperature What are catalysts? Catalysts are substances that change the rate of a reaction without being used up in the reaction. Catalysts never produce more product – they just produce the same amount more quickly. energy (kJ) Ea without catalyst Different catalysts work in different ways, but most lower the reaction’s activation energy (Ea). Ea with catalyst reaction (time) 51 of 39 © Boardworks Ltd 2007 Effect of a catalyst on Maxwel Botlzmann Glossary Catalysts: true or false? Catalysts in industry Why are catalysts so important for industry? ● Products can be made more quickly, saving time and money. ● Catalysts reduce the need for high temperatures, saving fuel and reducing pollution. Catalysts are also essential for living cells. Biological catalysts are special types of protein called enzymes. 55 of 39 © Boardworks Ltd 2007 Page 76 The minimum amount of energy needed to start a reaction. Enthalpy level diagram Exothermic Endothermic Reactants products Heat energy Heat energy (KJ) (KJ) Products reactants Time time Pages 96-97 Reactants Products Exothermic Page 103 A catalyst increases the rate of a reaction by : 1- lowering the activation energy 2- providing an alternative pathway 3- providing the correct orientation 4- are not involved in the reaction. Page 105 The number of particles More particles having enough energy to react Ea with A catalyst Energy Ea without a catalyst Page 107 Number of particles These particles Have unsuccessful collisions Ea with Catalyst Area increases Ea without a catalyst When activation energy is lower; area under the cur ve increases Thus; MORE particles now have enough sufficient energy to react. Energy Measuring Reaction Rates Concentration changes in a reaction can be followed indirectly by monitoring changes in mass, volume and colour. ◎ Why is rate difficult to measure directly? ◎ Measured INDIRECTLY. We need to monitor something changing over time such as: Technique Apparatus/Notes Mass loss Using a gas syringe or inverted measuring cylinder filled with water When a gas is produced Reaction conducted on a balance…if it produces a gas the mass will decrease Colour change Measured using a colorimeter Only when we know if a reactant or product is colored pH Monitored using a pH probe Only when we know if there is an acid or a base Temperature change Not ideal as difficult to prevent heat loss (think calorimetry) We can’t use change in temperature Because some reactions and endothermic and others are exothermic 60 Collecting gas Common techniques for measurements What equipment is needed to investigate the rate of hydrogen production? Stop watch Or timer Stop watch The cotton allows the gas to escape But not any other matter to enter or leave Record the increase in the volume of the gas with time 61 Record the decrease in the mass of the contents with time Setting up rate experiments What equipment is needed to investigate the rate of hydrogen production? glass tube conical flask rubber connecter gas syringe rubber bung hydrochloric acid magnesium 62 of 39 © Boardworks Ltd 2007 How can rate of reaction be measured? Measuring the rate of a reaction means measuring the change in the amount of a reactant or the amount of a product. What can be measured to calculate the rate of reaction between magnesium and hydrochloric acid? magnesium + hydrochloric ¨ magnesium acid chloride + hydrogen ● The amount of hydrochloric acid used up (cm3/min). ● The amount of magnesium chloride produced (g/min). ● The amount of hydrogen product (cm3/min). 63 of 39 © Boardworks Ltd 2007 Steeper graph Faster reaction Both will end with the same Amount of products How does temperature affect rate? The reaction between sodium thiosulfate and hydrochloric acid produces sulfur. sodium thiosulfate Na2S2O3 (aq) sodium + hydrochloric acid ¨ chloride + 2HCl (aq) 2NaCl ¨ (aq) + sulfur dioxide + SO2 (g) + sulfur + S (s) + water + H2O (l) Sulfur is solid and so it turns the solution cloudy. How can this fact be used to measure the effect of temperature on rate of reaction? 66 of 39 © Boardworks Ltd 2007 How does temperature affect rate? The reaction between sodium thiosulfate and hydrochloric acid produces sulfur. 67 of 39 © Boardworks Ltd 2007 The effect of temperature on rate 68 of 39 © Boardworks Ltd 2007 Reaction between a carbonate and acid Marble chips are made of calcium carbonate. They react with hydrochloric acid to produce carbon dioxide. calcium carbonate CaCO3 (aq) + + hydrochloric ¨ acid 2HCl (aq) ¨ calcium chloride CaCl2 (aq) + water + H2O (aq) + carbon dioxide + CO2 (g) The effect of increasing surface area on the rate of reaction can be measured by comparing how quickly the mass of the reactants decreases using marble chips of different sizes. 70 of 39 © Boardworks Ltd 2007 The effect of surface area on rate Faster reaction Slower reaction Interpreting Rate Graphs What does it mean if it is steeper? A bigger change is occurring per unit time therefore rate is increasing. Fastest reaction will have a steeper cur ve; tending more towards the y-axis 73 Lowest concentration will have a lower rate (slowest) ; least steep; Highest pressure for gas; fastest rate Rates of reaction: summary Higher Higher faster Higher 79 of 39 © Boardworks Ltd 2007 Refer back to Maxwell Boltzman distribution True True True The only factor that affects the activation energy is the catalyst Flase True