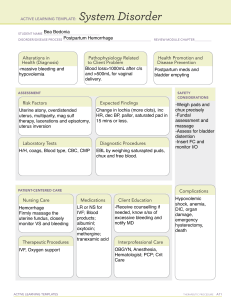
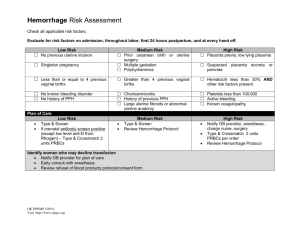
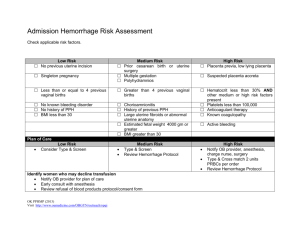
POSTPARTUM HEMORRHAGE Prepared by Dr. Hani Mahdi Modified Nov. 2019 Definitions: • ACOG defines postpartum hemorrhage as either of (regardless of mode of delivery) • cumulative blood loss ≥ 1,000 mL includes intrapartum loss • blood loss accompanied by signs or symptoms of hypovolemia within 24 hours after birth •Traditionally Postpartum hemorrhage traditionally defined as blood loss • > 500 mL after vaginal delivery • > 1,000 mL after cesarean delivery • Other definitions include • blood loss after delivery that causes hemodynamic instability • 10% decline in hematocrit after delivery Definitions: cont. •The Royal College of Obstetricians and Gynaecologists defines PPH as • minor: blood loss 500-1,000 mL • major: blood loss > 1,000 mL, further divided into • moderate (blood loss 1,000-2,000 mL) • severe (blood loss > 2,000 mL) •blood loss of > 40% considered life-threatening (about 2,800 ml in a 70 kg woman) •Postpartum hemorrhage can be further characterized as • primary: excessive blood loss in the first 24 hours after delivery • secondary: excessive blood loss 24 hours to 12 weeks after delivery Incidence •Primary postpartum hemorrhage 7% to 25% •Secondary postpartum hemorrhage reported in about 1% of pregnancies Complications of PPH • Postpartum hemorrhage reported to account for about 140,000 maternal deaths annually worldwide • Between 2003 and 2009, PPH was directly responsible for 20% of maternal deaths worldwide and 8% of maternal deaths in high-income countries. Potential complications of PPH: • Adult respiratory distress syndrome • shock, disseminated intravascular coagulation • acute renal failure • loss of fertility • pituitary necrosis (Sheehan syndrome) Less severe clinical outcomes: • iron deficiency anemia • fatigue • delayed lactogenesis Maternal Physiology: Cool Facts • Blood volume • 60 kg gravid women about 6 L by 30 weeks • Uterus weight • Pre pregnancy: 40 – 70 grams • Third trimester: 1,200 grams • Uterine cavity capacity • Pre pregnancy: 10 mL • Third trimester: 5,000 mL • Blood Flow • Pre pregnancy: 2% cardiac output • Third trimester: 17% cardiac output: 600 – 800 mL/min In postpartum women, it is important to recognize that the signs or symptoms of considerable blood loss (eg, tachycardia and hypotension) often do not present or do not present until blood loss is substantial Trauma Assessment of Blood Loss Class Blood Loss Volume Total Deficit Signs/Symptoms I <1000 mL 15% II <1500 mL 15-25% Resting tachycardia, orthostatic hypotension III <2,500 mL 25-40% Resting hypotension, oliguria IV >2,500 mL >40% Orthostatic Tachycardia Obtunded, Cardiovascular collapse RISK FACTORS ASSOCIATED WITH PPH • PPH often occurs in the absence of risk factors. •Researchers have identified numerous antenatal and intrapartum factors associated with increased risk of PPH. Most factors are not strongly predictive of PPH. • Risk assessment tools are readily available and have been shown to identify 60–85% of patients who will experience a significant obstetric hemorrhage. • A maternal risk assessment should be conducted antenatally and at the time of admission and continuously modified as other risk factors develop during labor or the postpartum period Admission Risk Assessment & Testing Low Medium High (Clot only) (Type and Screen) (Type & Crossmatch) No previous uterine incision Prior cesarean birth(s) or Placenta previa, low lying uterine surgery placenta Singleton pregnancy Multiple gestation Suspected placenta accreta, percreta, increta ≤4 previous vaginal births >4 previous vaginal births Hematocrit <30 AND other risk factors No known bleeding disorder Chorioamnionitis Platelets <100,000 No history of PPH History of previous PPH Active bleeding (greater than show) on admit Large uterine fibroids Known coagulopathy *Pre-transfusion testing strategy should be standardized to facility conditions depending on blood bank resources, speed of testing, and availability of blood products.* Ongoing Risk Assessment: At least q shift and at every handoff During Labor •Prolonged second stage •Prolonged oxytocin use •Active bleeding •Chorioamnionitis •Magnesium Sulfate treatment Birth/Postpartum •Vacuum- or forceps-assisted birth •Cesarean birth (especially urgent/emergent cesarean) •Retained placenta Reference: American Congress of Gynecologists and Obstetricians. Obstetric hemorrhage bundle Causes: Primary postpartum hemorrhage us ually caused by one or more of "the 4 Ts" (tone, tissue, trauma, or thrombin) 1- Uterine Atony (tone) 70% • Tone - abnormalities of uterine contraction (uterine atony) • Most common cause of primary PPH (70%) • Atony can be caused by: • overdistension of uterus • uterine muscle exhaustion • intra-amniotic infection • functional or anatomic distortion of uterus • uterine-relaxing medications • bladder distension, which may prevent uterine contraction •Risk factors for Uterine Atony •Placenta previa •Multiple gestation •Previous postpartum hemorrhage •Polyhydramnios •Macrosomia (neonate > 4000 g) •Rapid labor •Prolonged labor > 12 hours •Induction of labor •Oxytocin use •High parity •Age > 40 years (not multiparous) 2- Trauma 20% • Damage within or outside of the genital tract, including lacerations of the cervix, vagina, or perineum (including episiotomies with or without extensions) • Extensions, lacerations at cesarean section • Uterine rupture • Uterine inversion • Hematoma, including broad ligament hematoma • Extragenital bleeding, such a subcapsular liver rupture • Risk Factors for Trauma of the Genital Tract: • Delivery by cesarean section • Assisted vaginal delivery • Episiotomy, especially mediolateral • Precipitous delivery • Malposition • Previous uterine surgery • High parity • Excessive cord traction 3-Tissue 10% • Retained products of conception such as placenta or membrane, including abnormal placentation (especially placenta accreta) • Retained cotyledon, or succenturiate lobe • Retained blood clots • Risk factors for Retention of Tissue or Products of Conception: • Previous uterine surgery • High parity • Atonic uterus (due to retained blood clots) • Abnormally adherent placenta 4- Thrombin 1% •Abnormalities of coagulation, including pre-existing conditions, such as • hemophilia A • von Willebrand disease •Therapeutic anticoagulation •Conditions acquired in pregnancy, such as: • idiopathic thrombocytopenic purpura • thrombocytopenia, often due to preeclampsia with HELLP syndrome • Disseminated intravascular coagulopathy (DIC) • Placental abruption • Amniotic fluid embolus • Sepsis • Prolonged intrauterine fetal demise Secondary Postpartum Hemorrhage •Often idiopathic •Retained products of conception •Subinvolution of placental site •Infection (postpartum endometritis) •Inherited coagulation defects, such as von Willebrand disease Prevention • Active management of the third stage of labor: 1) oxytocin administration 2) Delay cutting the cord 3) umbilical cord traction (controlled cord traction) 4) Uterine massage • Prophylactic oxytocin, by dilute intravenous infusion (bolus dose of 10 units), or intramuscular injection (10 units), remains the most effective medication with the fewest adverse effects • Administration of uterotonics (usually oxytocin) after all births for the prevention of postpartum hemorrhage is recommended by WHO, ACOG, AAFP, Association of Women’s Health and Obstetric and Neonatal Nurses PLANNING • Management of risk — Identify and counsel women with risk factors for PPH • PPH protocols — Ideally, each hospital labor and delivery unit should have a PPH protocol for patients with estimated blood loss exceeding a predefined threshold (often 1000 mL). • The protocol should provide a standardized approach to evaluating and monitoring the patient with PPH, notifying a multidisciplinary team, and treatment. • PPH kits - kits including medications and instruments that may be needed to manage PPH so that this equipment is readily available when needed • Training and simulation —team training, clinical drills and debriefings after PPH Management of PPH due to Uterine Atony Treatment options for PPH because of uterine atony include administration of: • Uterotonics or pharmacologic agents • Tamponade of the uterus (eg, intrauterine balloons), • Surgical techniques to control the bleeding (eg, the B-Lynch procedure), embolization of pelvic arteries or, ultimately, hysterectomy. Uterine Atony • The bladder should be emptied • A bimanual pelvic examination conducted, any intrauterine clots should be removed, and uterine massage should be performed. • Bimanual compression • Oxytocic agents – Oxytocin – Prostaglandins – Methylergonovine • In addition to oxytocin, a second uterotonic agent is required in 3–25% of cases of postpartum hemorrhage • It is common for multiple uterotonic agents to be used, assuming there are no contraindications Acute Medical Management of Postpartum Hemorrhage Tranexamic Acid •Tranexamic acid should be considered in the setting of obstetric hemorrhage when initial medical therapy fails. •Earlier use is likely to be superior to delayed treatment (sooner than 3 hours from the time of delivery). Uterine Atony cont. • Treatment of refractory atony: •Use secondary methods such as uterine tamponade with an intrauterine tamponade balloon •Compression sutures Surgery and procedures: • Surgical intervention recommended if bleeding is refractory to uterotonics and other conservative interventions • For women with secondary postpartum hemorrhage and excessive or continuing bleeding, surgical treatment is necessary regardless of ultrasound findings TEMPORIZING MEASURES • External aortic compression: • Apply pressure with a closed fist on the abdominal aorta slightly to the patient's left and immediately above the umbilicus. Surgery and procedures: •Uterine tamponade: •Tamponade options include •Bimanual uterine massage/compression: • Place 1 hand in vagina and push up against body of uterus while other hand compresses fundus from above through abdominal wall. •Massage anterior aspect of uterus with abdominal hand and posterior aspect with vaginal hand Surgery and procedures: cont. • Uterine balloon tamponade : (condom catheter, Foley catheter, and Sengstaken–Blakemore esophageal tube): Foley catheter (insert ≥ 1 bulb and instill with 60-80 mL of saline) SOS Bakri tamponade balloon • insert balloon and instill with 300-500 mL of saline • can be left in place for 8-48 hours • prior to removal, deflate balloon but leave in place in case bleeding recurs • Uterine sandwich (a combination of external compression with internal tamponade) Tamponade Techniques for Postpartum Hemorrhage Cook “Bakri” Intrauterine Balloon ■ There are now several balloons, but the most known is the Cook “Bakri” Balloon ◻ ◻ ◻ ◻ ◻ ◻ ◻ Specifically designed for this purpose Double lumen (for drainage from above) Silicone (non-latex) Uterine contour shape Good filling capacity (saline) Inexpensive Easy to use Applications of Intrauterine Balloons • Low-lying placental implantation site (esp. placenta previa) • Poorly contracting lower uterine segment • Uterine atony • Placenta accreta / percreta • Cervical implantation • DIC at term or after 2nd trimester loss • In combination with Compression Suture at hysterotomy (“Sandwich technique”) • Vaginal sidewall lacerations • Easily placed when in stirrups for good exam for vaginal or cervical lacerations and for retained placenta Intrauterine Balloon Should be First Step after Failure of Medical Therapy •High success rate not different than other approaches •Low-tech, fast, inexpensive, easy to utilize on any L&D Unit •Least morbidity of any “next step” •Can be used as “Tamponade Test” to temporize, determine needs and mobilize other resources Issues for Balloons •There is some user learning— •How much to fill? (150-500 mL is a big range) --usually 250-300 mL is sufficient unless the uterus is very “floppy” •There can be “hour-glassing” of the balloon thru the cervix into the vagina. •So it is recommended to use vaginal packing if the cervix is more than 1-2cm dilated. Uterine Artery Embolization • Candidates for UAE: • Hemodynamically stable, • Persistent slow bleeding • Failed less invasive therapy (uterotonic agents, uterine massage, uterine compression, and manual removal of any clots). • Embolization with absorbable gelatin sponges, coils, or microparticles. • Studies (n=15) have shown that UAE for postpartum hemorrhage has a median success rate of 89%, ranging from 58% to 98% • After UAE, infertility has been reported in up to 43% of women • The risk uterine necrosis, deep vein thrombosis, or peripheral neuropathy appears to be less than 5% Surgical Management • Exploratory laparotomy is indicated when: • Uterotonic agents (with or without tamponade measures) or UAE fail to control bleeding. • The general aim of vascular ligation is to diminish the pulse pressure of blood flowing to the uterus. • A common first approach is bilateral uterine artery ligation (O’Leary sutures) • To further diminish blood flow to the uterus, sutures also can be placed across the vessels within the utero–ovarian ligaments. • When used as a second-line approach to PPH, the median success of vascular ligation is 92% Internal iliac artery ligation Internal iliac (hypogastric) artery ligation is performed less frequently The procedure has been found to be considerably less successful than originally thought Surgical Intervention • Uterine compression sutures:((Hemostatic Brace Sutures) • Suture techniques include B-lynch technique • Hayman suture -modified compression suture may be performed without hysterotomy • Cho Technique • Test for Uterine Compression Sutures • An assistant stands between the legs of the patient to determine the extend of bleeding. • Then the uterus is exteriorized and bimanual compression is performed. • The test is positive if the bleeding stops and the compression sutures will stop the bleeding Uterine Compression Sutures •The B-Lynch technique is the most common, other techniques, such as Cho and Hayman, have been described •The effectiveness appears to be approximately 60–75%, with none of the techniques shown to be superior to another •Similar effectiveness with uterine balloons •The suture should be rapidly absorbed to prevent risk of bowel herniation through a persistent loop of suture after uterine involution. B-Lynch Suture •Every Obstetrician should know how to do this (diagrams are in each OR) •Quick (<2 minutes) and easy! •Ideal at time of Cesarean birth for atony •Can be combined with an intrauterine balloon for “Sandwich technique” Perform B-Lynch within an hour •Among 211 women treated with B-Lynch sutures •Hysterectomy rate was 16% if done within an hour of delivery •Hysterectomy rate was 42% with a delay of 2-6 hours Move along a plan! Kayem G, Kurinczuk JJ, Alfirevic Z, et al. Uterine compression sutures for the management of severe postpartum hemorrhage. Obstet Gynecol. Jan 2011;117(1):14-20. B-Lynch Suture completed Photo courtesy of Elliott Main, MD and used with permission Hayman stitch Two to four vertical compression sutures are placed, as needed, but in contrast to the B-Lynch technique, these sutures pass directly from the anterior uterine wall to the posterior uterine wall. A transverse cervicoisthmic suture can also be placed if needed to control bleeding from the lower uterine segment. Cho Technique #1 chromic catgut is used to place sutures in a small rectangular array to compress the anterior and posterior uterine walls against one another at sites of heavy bleeding. The through and through sutures extend from the serosa of the anterior wall to the serosa of the posterior wall. After creating a square, the ends are tied down as tight as possible to compress the myometrium. Two to five squares/rectangles are made, as needed. Hysterectomy •When more conservative therapies have failed, hysterectomy is considered the definitive treatment •In the setting of an emergent postpartum hysterectomy, the surgical approach felt to be the fastest and safest should be used. •Inadequate evidence examining different surgical approaches to hysterectomy (eg, total hysterectomy versus supracervical hysterectomy) •Regardless of the patient’s wishes for the avoidance of hysterectomy, this procedure may be necessary in a life-threatening situation Hysterectomy cont. •Potential indications for hysterectomy include: Placenta accreta or placenta previa (most common) Uterine rupture Atony, after other management attempted Trauma Sepsis •Potential surgical complications: •Bladder injuries range from 6% to 12% •Ureteral injuries range from 0.4% to 41% Obstetric Trauma • Rapid identification and repair of cervical lacerations, lacerations complicated by arterial bleeding, and high vaginal lacerations should be performed. • Distal vaginal, vulvar, periclitoral, and perineal lacerations should be repaired if contributing significantly to blood loss. • Genital tract hematomas can lead to significant blood loss and should be suspected in the setting of a precipitous uncontrolled delivery or an operative vaginal delivery. • Once identified, most genital tract hematomas can be managed conservatively. However, rapid progressive enlargement of the hematoma, particularly in the setting of abnormal vital signs, indicates a need for incision and drainage. Exploration with suturing or packing may be needed to achieve hemostasis. • Deterioration of maternal vital signs without obvious bleeding should alert the obstetric team that there may be intraperitoneal or retroperitoneal bleeding. In this setting, resuscitative measures, diagnostic imaging, and surgical intervention or an interventional radiology procedure should not be delayed. Surgery for hematomas: •For postpartum hemorrhage due to genital tract hematoma, incision and drainage is necessary with suturing of the incision, and if appropriate, packing the vagina •genital tract hematomas can lead to significant blood loss •progressive enlargement of the mass indicates need for incision and drainage •≥ 1 source of bleeding is often identified after incision •hemostasis is usually achieved by: •draining the blood within the hematoma (if necessary place drain in situ) •packing the vagina when appropriate Management approach for hemorrhage due to a ruptured uterus • Assess rupture via exploratory laparotomy to determine best surgical option, including • primary repair (rupture of previous cesarean section scar can often be successfully repaired by revision of the edges of prior incision followed by primary closure) • hysterectomy (may be necessary in a life-threatening situation) • Type of surgery performed dependent on: • site and extent of rupture • patient's clinical condition • patient's desire for future childbearing • reconstruction of the uterus, if possible • neighboring structures, such as broad ligament, parametrial vessels, ureters, and bladder Retained Placenta • Detailed visual inspection of the placenta for completeness should be conducted after all deliveries. • Manual removal of the placenta, prior uterine surgery, or other risk factors for morbidly adherent placenta should raise suspicion for retained placental tissue or placenta accreta. • Ultrasonography or intrauterine manual examination is usually used to diagnose retained placental tissue. • When a retained placenta is identified, the first step is to attempt manual removal of the tissue. If manual extraction fails, either a “banjo” curette or large oval forceps (Sopher or Bierer) can be used for removal. • Because of the concern for uterine perforation in the postpartum uterus and to ensure removal of all tissue, ultrasound guidance may be used Clinical considerations for placenta accreta • The risk of placenta accrete is increased with the number of cesarean deliveries (ie, 0.2%, 0.3%, 0.6%, 2.1%, 2.3%, and 6.7% for women experiencing their first through sixth cesarean deliveries, respectively) • The risk is higher in women with placenta previa with 3%, 11%, 40%, 61%, and 67% of such women with their first through fifth or more cesarean deliveries having a placenta accrete. • An organized, multidisciplinary management and delivery plan should be developed: • Establishing a delivery date • Assembling an experienced team (including surgical, anesthesiology, blood bank, nursing, and neonatal intensive care unit personnel) and relevant resources (including an operating room and equipment) Placenta accrete cont. • In the setting of postpartum hemorrhage and a vaginal delivery, accreta should be strongly suspected if the placenta does not detach easily • The patient should be counseled about the need for hysterectomy and blood transfusion. • In the operating room, the extent (eg, area and depth) of the abnormal attachment can be assessed to determine the plan (eg, curettage, wedge resection, medical management, or hysterectomy). Placenta accrete cont. • Uterine conserving options may work in a small focal accrete with a clear understanding of the significant risks of this approach • Uterine conservation is associated with a 40% risk of emergency hysterectomy, and 42% of women suffered major morbidity. The risk of an abnormally adherent placenta in a subsequent pregnancy is 20% • A prompt hysterectomy should be underway in accreta with ongoing bleeding • Blood products (including red blood cells, fresh frozen plasma, platelets, and cryoprecipitate) should be made readily available • Other specialties such as urology, surgery, or interventional radiology should be notified in case additional support is needed. Acute Coagulopathy • Two specific etiologies beyond massive blood loss alone should be considered: 1) Placental abruption • often is associated with uterine atony. DIC and hypofibrinogenemia are known complications. • responsible for 17% of cases that require massive transfusion. 2) Amniotic fluid embolism. • rare, unpredictable, unpreventable obstetric emergency signaled by a triad of hemodynamic, respiratory compromise and DIC. Management of Coagulopathy • Treat underlying disease process • Serially evaluate coagulation status • Replace appropriate blood components • Support intravascular volume Coagulopathy should be managed with aggressive volume replacement and initiation of a massive transfusion protocol Uterine Inversion • Rare • Important to recognize quickly • Suspect if shock disproportionate to blood loss • Replace uterus immediately • Watch for vasovagal reflex Uterine relaxants may help restore normal anatomy (terbutaline) Manual replacement with or without uterine relaxants is usually effective • procedure involves digital application of upward pressure against inverted fundus • in case of inversion before separation of placenta, do not remove placenta until uterus has been manually put back into position Laparotomy required if failed manual replacement, using • Huntington procedure (Application of atraumatic clamps to each round ligament and upward traction) Haultain procedure (a sagittal surgical cut is made in cervical os posteriorly through the muscular ring to release it followed by digital replacement of corpus and repair of incision Uterine Inversion Manual Replacement Obstetric Hemorrhage Safety Bundle •Readiness •Recognition •Response •Reporting / Systems Learning Photo courtesy of David Lagrew, MD and used with permission Obstetric Hemorrhage Safety Bundle Readiness: (every unit) • Hemorrhage Cart / with Procedural Instructions (balloons, compression stiches) • Rapid access to hemorrhage medications (kit or equivalent) • Establish a response team: multiple partnerships // unit education, drills, debriefs • Establish MTP and 0-neg/uncrossmatched transfusion protocols Recognition: (every patient) • Assessment of hemorrhage risk (prenatal, on admission, ongoing in labor & PP) • Measurement of CUMMULATIVE blood loss • Active Management of 3rd Stage (oxytocin after birth) Obstetric Hemorrhage Safety Bundle Response: (every hemorrhage) • Unit-standard, stage-based OB Hemorrhage Emergency Management Plan with checklist • Support program for patients, families and staff Reporting / Systems Learning: (every unit) • Establish a culture of Huddles for high-risk patients and post-event debriefings • Monitor outcome and process metrics in perinatal QI committee Transfusion and Massive Obstetric Hemorrhage •Massive transfusion usually is defined as • transfusion of ≥ 10 red blood cell (RBC) units within 24 hours • transfusion of > 4 RBC units in 1 hour with anticipated need for further blood product transfusion • replacement of > 50% of total blood volume (TBV) by blood products within 3 hours • The recommended initial transfusion ratio for packed red blood cells: fresh frozen plasma:platelets has been in the range of 1:1:1 and is designed to mimic replacement of whole blood. • Administration of cryoprecipitate also should be considered in the setting of placental abruption or amniotic fluid embolism(where fibrinogen is low) • In emergency situations, type specific or type O Rh-negative blood also should be readily available. General principles of management The goal is to: •Restore or maintain adequate circulatory volume to prevent hypoperfusion of vital organs •Restore or maintain adequate tissue oxygenation •Reverse or prevent coagulopathy •Eliminate the obstetric cause of PPH Treatment overview: •Use team approach •Initiate full resuscitative measures for postpartum patients with estimated blood loss ≥ 1,000 mL and continued bleeding, or signs of hypovolemic shock (activation of a massive transfusion protocol). •Call experienced medical staff (Obstetrician, midwife, Anesthesia, Hematologist, and Laboratory). •Alert one member of the team to record event. •Don’t forget communication with patient and her family Immediate action •Provide intravenous fluid and possibly blood replacement by starting two large bore IV’s with normal saline or other crystalloid fluids. •Elevating the foot of the bed or having an assistant elevate the patient’s legs will improve venous return and raise the patient’s blood pressure. •Open the airway and give supplemental oxygen to maintain oxygen saturation of greater than 95 percent. • Ventilate the patient if needed, with 100 percent oxygen. Frequently monitor •Fluid replacement and use of blood products •Vital signs, especially temperature and blood pressure as they are more often affected with shock •CBC and coagulation studies •The prevention of hypothermia and acidosis is an essential component in the successful management of massive hemorrhage. •Patients should be kept warm. All fluids should be warmed using any of the commonly available fluid warmers •Identify and treat source of bleeding Measurement of blood loss •Cumulative measurement of blood loss at every delivery. •Collect blood in graduated measurement containers •Use visual aids (eg, posters) that correlate the size and appearance of blood on specific surfaces (eg, maternity pad, bed sheet, lap sponge) •Measure the total weight of bloody materials and subtract the known weight of the same materials when dry. •Bedside evaluation by the provider. Blood tests: •Blood type and cross match recommended in case transfusion is needed • Complete blood count (CBC) and coagulation studies should be obtained and repeated periodically as clinically needed •Coagulation studies should include: • prothrombin time • partial thromboplastin time •Fibrinogen assay Blood tests cont. •Bedside evaluation of clotting status may be undertaken on recently lost blood using the clot observation test to rule out coagulopathy •5 mL of patients blood placed in clean redtop tube •Normal blood clots within 8-10 minutes and remains intact •Low fibrinogen concentration indicated by: - failure to clot, - or partial or complete dissolution of clot within 30-60 minutes Fluid and Blood Products Transfusion in PPH Fluid and electrolytes: •For patients with minor hemorrhage (blood loss 500-1,000 mL) and no clinical signs of shock include: •establishment of IV access ( large bore canula) •crystalloid IV infusion •In women with major hemorrhage (blood loss > 1,000 mL and continued bleeding, and/or clinical shock), infuse maximum of 3.5 L of clear fluids while awaiting compatible blood Blood products: •Blood transfusion indicated with major postpartum hemorrhage (blood loss > 1,000 mL) •Purpose: to replace coagulation factors and red cells for oxygen-carrying capacity, not for volume replacement • British Committee for Standards in Hematology (BCSH) recommends that the main therapeutic goals of management of massive blood loss is to maintain • hemoglobin > 8 g/dL • platelet count > 75 x 109/L • prothrombin < 1.5 x mean control • activated prothrombin times < 1.5 x mean control • fibrinogen > 1.0 g/L Blood products cont. •Blood component therapy: • packed red cells 240 mL (red cells, white cells, and plasma) to increase hematocrit 3 percentage points and hemoglobin by 1 g/dL per unit • platelets 50 mL (platelets, red cells, white cells, and plasma) to increase platelet count 5,000-10,000 mm3 per unit • Concurrent replacement with coagulation factors and platelets to avoid dilutional coagulopathy, including: • fresh frozen plasma 250 mL (fibrinogen, antithrombin III, and factors V and VIII) to increase fibrinogen by 10 mg/dL per unit • cryoprecipitate 40 mL (fibrinogen, factors VIII and XIII, von Willebrand factor) to increase fibrinogen by 10 mg/dL per unit Complications of transfusion • Massive transfusion is associated with hyperkalemia from packed red blood cells and citrate toxicity that will typically worsen hypocalcemia. • Overzealous resuscitation with crystalloid also can be associated with dilution-related coagulopathy and can contribute to pulmonary edema • Other complications include transfusion febrile nonhemolytic reactions (0.8 per 1,000 units transfused), acute hemolytic transfusion reaction (0.19 per 1,000 units transfused), and acute transfusion reactions related lung injury (TRALI, 0.1 per 1,000 units transfused) Common mistakes made in the assessment and management of PPH 1. Underestimating blood loss 2. Delay in noticing vital sign trends 3. Delay in laboratory assessment for anemia and coagulopathy 4. Delay in instituting blood component therapy 5. Delay in surgical interventions 6. Delay in making the mental shift from normal delivery to life-threatening emergency 7. Poor communication between the OB, nurse, and anesthesiologist 8. Lack of preoperative preparation for massive hemorrhage Management of PPH: organizing the team (Pingirl) Take a Home Message