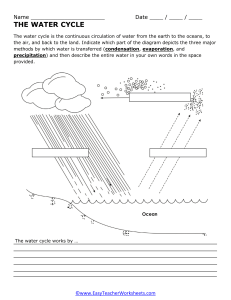
EXPLAINING THE CHANGES OF STATE OF MATTER Kinetic Theory of Particle 1. When a substance changes from a solid to a liquid, what is this process called? a) Condensation b) Evaporation c) Melting d) Sublimation 2. Which of the following is a change from a gas to a liquid state? a) Evaporation b) Condensation c) Freezing d) Melting 3. During which change of state do particles gain energy and move more freely? a) Sublimation b) Melting c) Condensation d) Freezing 4. What is the process by which a liquid turns into a gas at its boiling point? a) Condensation b) Evaporation c) Sublimation d) Freezing 5. In which change of state does a substance directly go from a solid to a gas without becoming a liquid first? a) Melting b) Sublimation c) Evaporation d) Condensation True or False: 6. True or False: Sublimation is the process of changing from a liquid to a gas. 7. True or False: Evaporation is a faster process than condensation. 8. True or False: In a change of state, the substance's chemical composition remains the same. 9. True or False: In the water cycle, evaporation occurs when water vapor turns into liquid water. Fill in the Blanks: 10. During ___________, a substance changes from a gas to a liquid. 11. The process of a liquid turning into a gas below its boiling point is called ___________. 12. In ___________, a substance changes from a solid to a gas without becoming a liquid first. Short Answer: 13. Explain the difference between condensation and evaporation, including when and where these processes typically occur. 14. Provide an example of a substance that undergoes sublimation. How is this change of state used in real-life applications? 15. Describe the role of changes of state in the water cycle and how they influence weather patterns. Application: 16. Design an experiment to demonstrate the process of evaporation and condensation. List the materials and steps you would use. 17. Research and explain how changes of state are used in food preservation or manufacturing processes. Critical Thinking: 18. Discuss the significance of understanding changes of state in fields such as meteorology, climate science, and cooking. 19. Consider a scenario in which sublimation could be used to solve a real-world problem. Describe this scenario and explain how sublimation would be applied.