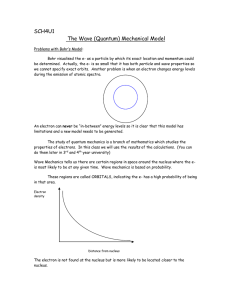
Why Quantum Mechanics? • Inadequacy of classical mechanics in explaining certain experimental observations [stability of atoms, blackbody radiation, etc.] • Need for a new theory to explain phenomena on the atomic scale • Quantum mechanics was able to successfully explain such events • Useful for explaining the structure of atoms and molecules • Reduces to classical mechanics when we deal with large dimensions compared to atomic distances i.e., classical mechanics is a special case of a more general principle of quantum mechanics • Difficult to visualize the quantum behavior unlike the classical events Blackbody Radiation • Max Planck (1901) • Black body: absorbs all incident radiation • Spectrum of the emitted radiation approximately the same for all solids at a given temperature and approaches black body spectrum • Planck’s assumptions: – Energy of an oscillator, E = nh – Transition from one level to other associated with the emission or absorption of energy – Energy lost or gained in the transitions is discrete Photoelectric Effect • Einstein (1905) • Light incident on a metal surface causes ejection of electrons from the metal surface • Classical explanation: – Electrons leave the metal surface only if they have sufficient energy to overcome the potential barrier at the surface, W called the work function. W is a constant for a given chemically clean metal surface – Kinetic energy imparted to the electron was a function of the intensity of the radiation. – As such, a lower intensity limit would exist below which no electron emission could take place – Could not explain the observed behavior Photoelectric Effect • Einstein’s assumptions: – Incident radiation made up of discrete pulses called photons – Photons are absorbed or given off in discrete amounts – Energy of photon is an integral multiple of h – Photons behave like waves of corresponding frequency Em o W A I2 I1 A + V Vs V h = W + Em Em = h - W Hydrogen Spectra • Niels Bohr (1913) • Model to explain and predict the hydrogen spectra • Experimental observations: – Every atom has a unique spectra characteristic of the atom – Plot of intensity of the emitted light as a function of the wavelength gives a series of sharp lines rather than a continuous distribution of wavelengths – These lines appear in several groups called Lyman, Balmer and Paschen etc. – The various series in the spectrum were found to follow the following empirical forms where R is a constant called the Rydberg constant. 1 1 Lyman : cR 2 2 , n 2,3,4,... 1 n 1 1 Balmer : cR 2 2 , n 3,4,5,... n 2 1 1 Paschen : cR 2 2 , n 4,5,6,.... n 3 Bohr’s Model • Bohr’s postulates: – Electrons exist in certain stable, circular orbits about the nucleus – Electron may shift to an orbit of higher or lower energy by absorption or emission of a photon of energy h – Angular momentum of the electron in an orbit is always an integral multiple of Planck’s constant • Bohr’s radius – For an electron in an orbit of radius r around the proton in the nucleus, q2 mv 2 2 4 0 r r From the third postulate, mvr n This gives, 4 0 n 2 rn mq 2 2 Bohr’s Model • Expression for energy in Bohr’s orbits: Total energy of the electron in the nth orbit, 2 1 q E n K.E. P.E. mv 2 2 4 0 rn where v n / rn mq 4 mq 4 2 2 2 2(4 0 ) n (4 0 ) 2 n 2 2 mq 4 2(4 0 ) 2 n 2 2 Now, the energy difference between different levels n1 and n2 , 1 mq 4 1 2 2 En En 2 2 2(4 0 ) n 1 n2 2 1 Principles of Quantum Mechanics • Probability and the Uncertainty Principle – Probabilistic nature of events involving atoms and electrons – Impossible to describe with absolute precision events involving particles on the atomic scale – Uncertainty in quantum calculation not result of any shortcoming of the theory – The magnitude of the inherent uncertainty described by the Heisenberg uncertainty principle: • (x)(p) • (t)(E) – We cannot speak of finding a particle at a particular position – Expected values of position, momentum, energy etc. are relevant • Wave nature of atomic particles – de Broglie equation (1924) – Relationship between wavelength and momentum, = h/p Schrodinger Equation • Postulates: – Each particle represented by a wave function (x,y,z,t) – Classical quantities like momentum, energy etc. replaced by quantum mechanical operators as follows: Location, x x Momentum, p( x ) Energy, E j x j t – Probability of finding a particle in a volume dx dy dz is * dx dy dz. The total probability of finding the particle anywhere should be one. So, the integral of * dx dy dz over the entire space is normalized such that * dxdydz 1 – The expectation value <Q> of any variable Q is calculated as, Q *Q op dx dy dz Schrodinger Equation From classical mechanics, the energy of a particle may be written as, E = K.E + P.E. = p2/2m + V Substituting for the relevant operators, for a particle in one dimension, 2 2 ( x, t ) ( x , t ) V ( x ) ( x , t ) 2m x 2 j t In three dimensions, the equation becomes 2 2 V 2m j t 2 2 2 where 2 x 2 y 2 z 2 Thus, the problem of Quantum mechanics reduces to the solution of the Schrodinger under the given boundary conditions. Time Independent Schrodinger Equation • • The wave function includes both space and time dependencies. It is easier to calculate these dependencies separately and then combine them together Suppose for one-dimensional Schrodinger equation, (x,t) = (x)(t) then 2 2 (x ) ( t ) ( t ) V ( x ) ( x ) ( t ) ( x ) 2m x 2 j t By separating the variables, we get the time-dependent equation in one dimension, d( t ) jE ( t ) 0 dt and the time-independent equation, • • d 2 ( x ) 2m 2 [E V( x )] ( x ) 0 dx 2 Many problems are time independent and so we need only space dependent solution We use the time independent Schrodinger equation when the property of atomic systems has to be calculated in stationary conditions Schrodinger Equation for a Free Particle We consider the case of a electron which moves freely in the positive x direction. The potential energy is zero and so the Schrodinger equation may be written as, 2 2m E 0 x 2 2 The solution of this equation is of the form, (x) = Aejkx + Be-jkx where k 2mE / But, as the electron is moving in positive x direction, we only need to take the first term which corresponds to the wave motion in positive direction. So, (x) = Aejkx 2 2 p2 Now , E k and also E 2m 2m h So, p k (from de Broglie equation ) 2 k and is called the wave vector. As the wave vector, k can take any values, all values of energies are allowed for a free electron as in the classical case. Criteria for Solution of Schrodinger Equation • Both and d/dx must be continuous at the boundaries. If it is not so, it would equivalent to creation or destruction of particles as * is proportional to the probability of finding the particle • must be single-valued as otherwise this would lead to more than one probability of finding the particle • should never be identical to zero as that would amount to zero probability of finding the particle anywhere in the system Infinite Potential Well • Simplest example • One dimensional problem • Potential defined as: V= – V(x) = 0 for 0 < x < L ( inside the well) – V(x) = elsewhere I • The Schrodinger equation for region II, V= II III x 0 L 2 ( x ) 2m 2 E ( x ) 0 2 x • The solution for this equation is of the form, ( x ) Ae jkx Be jkx where k 2mE / • As per the barrier is infinite, the particle cannot be found in these regions. So, the wave function must be zero in the region I and III as otherwise there will be a non-zero probability of finding the particle in these regions Infinite Potential Well • As the wave function is zero in region I and III, for the wave function to be continuous at x = 0 and x = L, II (0) = 0 and II (L) = 0 • II (0) = 0 B = -A and so (x)= Aejkx - Ae-jkx = 2jAsinkx • II (L) = 0 2jAsinkL = 0 k = n/L where n = 1,2,3,… (Solution with n =0 is not a possible solution as then (x) = 0 • This gives, n 2 2 2 En 2mL2 where n 1,2,3,... • This means that the electron inside an infinite potential well cannot have all energy values. It can only take discrete values. So, the energy is quantized. Infinite Potential Well • The constant A in the wave function can be found by normalizing the wave function, • This gives n 2 / L sin(n / L) x • Also, the probability of finding the particle inside the well is not uniform • When n becomes large, the probability of finding the particle becomes uniform as in the classical case. Finite Potential Barrier Problem: A free electron with energy, E moving in the positive x direction with a potential barrier of height V0 at x = 0 such that E < V0. V V0 I II x=0 x We have to write two different Scrodinger equations for region I and region II as the electron is free in the region I and the potential is V0 in region II. 2 2m 2 E 0 x 2 2 2m and in region II, 2 (E V0 ) 0 x 2 In region I, The solution to the Schrodinger equation in region I is , I Ae jkx Be jkx where 2mE / 2 Finite Potential Barrier The solution to the Schrodinger equation in region II is , I Cex De x where 2m( V0 E ) / 2 Now, as E < V0, (V0-E) would be positive and so is real. We determine the constants A ,B ,C and D by means of the boundary conditions. Now, at x= , II= . This means that the probability density II* II would become infinity. So, C0 i.e., II = De x At the boundary, x=0, the wave function and its derivative should be continuous. So, at x=0, I= II and d II/dx=d II/dx. This gives, A B D and Aj Bj D Solving these two equations, we get A D D (1 j ) and B (1 j ) 2 2 So, we get A and B in terms of D. We can solve for D by normalizing the wave function. Finite Potential Barrier The wave function in region II, II= De- x Thus, the wave function decreases exponentially in region II but has a nonnegative value inside the barrier. This has following implications: – In classical case, the electron would never enter the barrier as the energy of the electron is less than the potential barrier. – Now, the electron will have a non-zero probability of being found inside the barrier – If the potential barrier is high, the wave function will decrease very rapidly and will soon become zero – And if the barrier is only moderately high and is relatively narrow, the wave function will continue across the barrier on the other side (we have taken a barrier of very large width) – The quantum mechanical effect of penetration of a potential barrier by the electron is called “tunneling” and has important implications in solid state physics Hydrogen Atom • Solution of Schrodinger equation in three dimensions for the coulombic potential • The Coulomb potential is given by, q2 V(r ) 4 0 r • Use of spherical coordinates required as the potential is inversely proportional to the distance from the nucleus • The Schrodinger equation can be written in the polar coordinates as, 1 2 1 1 2 2m 2 E V r 0 r 2 sin 2 2 2 2 r r r r sin r sin • Solution of the form (r,,) = R(r)()() is obtained Hydrogen Atom • After the separation of variables, separate solution is found for the r-dependent, -dependent and -dependent equations and then the total wave function is obtained from the product. • The -dependent equation is of the form, • The solution to this • d 2 2 m 0 2 d equation is, 1 jm m () e 2 As value of varies between 0 and 2, the value of should repeat after every 2 for which m must be an integer i.e., m = ….,-2,-1,0,+1,+2,…. Hydrogen Atom • Similarly, solutions of r-dependent and -dependent equations are quantized and so each state is represented by three quantum numbers n, l and m. • The interrelationship between the equations leads to following restrictions: – Principal quantum number, n = 1,2,3…. – Azimuthal quantum number, l = 0,1,..,n – Magnetic quantum number, m = -l,-(l-1),..,-1,0,1,..,(l-1),l • Another quantum number, called spin quantum number required to explain the observed spectral behavior of hydrogen atom in non-homogeneous magnetic field. • This comes from quantization of intrinsic angular momentum of the electron. Spin quantum number, s can take values +1/2 and -1/2 Hydrogen Atom • Each allowed state of the electron in the hydrogen atom is uniquely described by the four quantum numbers n,l,m and s and is represented by nlms . • The principal quantum number, n corresponds to the orbit of electron in the Bohr model • The energy for the electron in the hydrogen atom is given by, given by, mq 4 En 322o2 2n 2 • Electron in the hydrogen atom can occupy any of the large number of excited states in addition to the lowest energy level 100 • Energy difference between various states properly accounts for the observed lines in the hydrogen spectra Periodic Table • Energy levels obtained by solution of the Schrodinger equation for the hydrogen atom cannot be extended to other atoms without appropriate modifications • Quantum number selection rules valid for complicated spectra and adequately explain the electronic structure of atoms • Pauli’s exclusion principle restricts the occupancy of same quantum state by more than one electron • This decides the maximum number of electrons occcupying a shell(orbit) • Maximum number of electrons in a shell - 2n2 • The different values of l denote orbitals and are represented as s (l = 0), p (l = 1), d (l = 2), f (l = 3) etc.