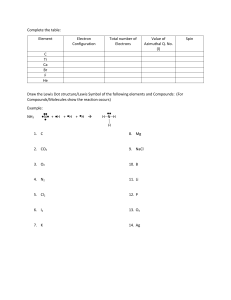
Name ____________________________ BONDING PRACTICE PROBLEMS DIRECTIONS: Illustrate the electron dot configuration for each of the following ionic compounds. Then write the ions that result due to ionic bonding. 1) LiF 4) CaF2 2) MgO 5) MgCl2 3) K2S 6) CaCl2 DIRECTIONS: For each of the covalently bonded compounds, illustrate the electron dot configurations for each element and bond. 1) HF 4) CH4 2) O2 5) H2S 3) NH3 6) CCl4