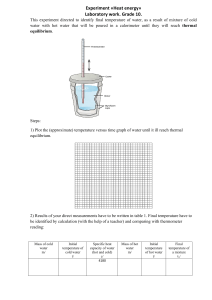
Assignment in General Chemistry 2 1. The reaction between gaseous sulfur dioxide and oxygen is a key step in the industrial synthesis of sulfuric acid: 2SO2(g)+O2(g)⇌2SO3(g) A mixture of SO2 and O2 was maintained at 800 K until the system reached equilibrium. The equilibrium mixture contained 15.0 × 10−2 M SO3, 7× 10−3 M O2, and 6.5 × 10−3 M SO2. Calculate Kc and Kp at this temperature. 2. At 430°C, 4.20 mol of HI in a 9.60 L reaction vessel reaches equilibrium according to the following equation: H2(g) + I2(g) ⇌2HI(g) At equilibrium, [H2] = 0.047 M and [HI] = 0.345 M. What is the Kc and Kp for this reaction?