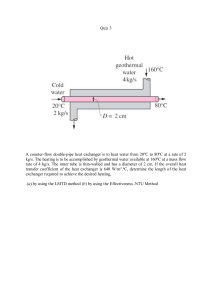
Learning unit 1: The design process Reading: To complete this unit, study chapter 1 in your prescribed textbook, Chemical engineering design. 1.1 Introduction The design process is a series of steps that engineers follow to come up with a solution to a specific problem. It could be thought of as a creative idea or synthesis of ideas that the engineer puts together in order to achieve the desired outcome. In any design process, organisation of ideas is needed which takes a set of input resources and transforms them into output products and/or solutions to a set of goals. The design engineer starts with a specific set of goals in mind, and by developing and evaluating different possibilities, arrives at the best way of achieving the goals. During the design process, the engineer will be faced with many constraining factors which would decrease the number of possible designs. As the design develops, they would be able to evaluate possible design solutions and explore other possibilities. Since the design process is likely to be an iterative one, the use of various tools such as applicable computer software is important. Figure 1.2 in the prescribed textbook highlights the iterative nature of the design process. In this learning unit, we discuss the key steps in the design process. We consider the various constraints that influence the design and explain the tools to evaluate and assess flow sheets. 1.2 Learning outcomes At the end of this learning unit, you should be able to evaluate design decisions that challenge the product and process design teams explain key steps in process design discuss the importance of environmental aspects, safety issues and ethics in the design process describe the tools required to design products and processes 1.3 Design opportunities The objective of the design process is to develop a safe, environmentally compliant and sustainable process scheme for manufacturing and/or processing raw materials into industrial resources or consumer products. Generally, the objective of the design process is to meet a specific societal need while generating profit for the enterprise undertaking the process development. The design process is fairly complex and is typically implemented in stages. Because of the need to draw in expertise on a range of problems, the design process is usually conducted within a team of subject area experts, drawing from experience in both technical and operational aspects of chemical plants. Typical stages in design development can differ depending on the type of product being produced. In that sense, 1 Open Rubric chemical feedstocks and associated products can be classified in three distinct categories as outlined in table 1.1. Table 1.1: Typical stages in the design process Categories of chemical feedstocks and associated products Example Natural resources being converted to basic chemical products Natural gas conversion to ethylene Basic chemical products being converted to industrial products Ethylene used to produce high‐density polyethylene film Basic chemicals and industrial products being converted to consumer products Polyethylene film used for manufacturing food packaging and shopping bags Since consumer products are designed to meet a specific application, product design reflects the need for a distinct set of properties provided by the chosen material of construction. When considering consumer products, these properties would often be physical in nature (such as colour, strength, porosity and permeability). On the other hand, physical properties of industrial products generally arise as a result of differences in chemical composition of the materials used to produce them. Therefore, typical designs for chemical plants would include product composition specifications. Chemical processes can differ significantly by scale and complexity. The conversion of natural resources into commodity chemicals and their conversion to industrial products are usually large‐ scale activities, which may involve processing millions of tons of product annually. On the other hand, manufacturing consumer goods, specialised chemicals and materials would generally be performed on a significantly smaller scale. Scale plays an important role in terms of expected design complexity, development costs and schedule, implementation/construction time, etc., which will ultimately have an impact on the kinds of decisions made throughout the process development cycle. Designs could also originate from different circumstances. Most common origins of new products are from the desire to improve on existing products already available in the market ‐ be it from the perspective of improving manufacturing efficiency by reducing production costs (and conversely, increasing profits to the manufacturer), or from the perspective of product differentiation (e.g. competition between similar products in niche markets). Product innovation implies understanding the potential market opportunities and being able to exploit specific technological levers not understood or available to competitors. Achieving technological advantage over competition is one of the imperatives of any modern enterprise, and research and development (R&D) departments play an important role in achieving that goal. 1.4 Steps in product and process design Design is an open‐ended problem, i.e. there are many solutions to the problem. It is the role of the process designer to explore the various alternatives and specify the most superior design. In many cases, this is not a simple task, as there are many objectives (usually conflicting) and constraints that 2 need to be considered. Usually, the designer designs a process that is most economical, but other factors, such as safety, environmental issues, location and social aspects, need to be taken into account as well. This makes the design an iterative process because often the designer may need to revisit previous design decisions as more data and ideas become available, which may have turned out to be unsatisfactory in terms of the constraints and objective set for the process. Many companies have developed their own distinct approach to process design development, often encompassing the collective experience of many different teams within the company, or within a specific industry. Process engineering techniques presented in this course are intended to give a generalised approach to process design development, which is applicable to most industries. The sequence of steps involved in the design, construction, start‐up and operation of the chemical process is shown in figure 1.1. Note that the sequence shown in figure 1.1 is rather idealised, since in most practical cases, the sequence of events may not be so clear‐cut due to the iterative nature of the design process. A blend of manual calculations, computer software, spreadsheets and process simulators are used in the design process. 1.4.1 Understand and explore The first step is to define the problem to solve. Without fully understanding the problem, how can an engineer solve it successfully? This step is often done incorrectly or incompletely and results in the failure of the design. It is important to define the true problem you are solving, not just the symptoms of the problem or the perceived problem. The following excerpt has been extracted from http://www.vexrobotics.com/. The example shows that once the problem is understood in context, a solution path is mapped out in a much easier way. It is also important to do thorough background research into the problem. Investigate the ways others have used to solve similar problems, take note of the environment being dealt with, the situation for which the solution is required and the way it will be used. 3 Case study: Understand and explore When trying to define the real problem, remember the elevator riddle, as follows. There is a story about a skyscraper which is told to young engineers to emphasize the importance of this step in the design process. There was a skyscraper in a major city and the occupants of the building were complaining that the elevator ride times were too long. The owners of the building wanted to fix this, so they put out a call to several local engineering firms asking them for proposals. 1. One firm put in a bid to renovate the office and add two additional elevators. They speculated that adding more elevators would cut down on elevator stops and decrease the average ride time. They estimated this would cost some ludicrous amount of money (details vary based on the telling.) 2. Another engineering firm suggested renovating the building and adding some brand new, state of the art, high‐speed elevators. These faster elevators would also reduce ride time. This suggestion didn’t cost as much as the first proposal, but was still a ridiculous amount of money. 3. The third engineering firm came back with a proposal to upgrade the elevator software. They claimed that they had devised a new algorithm that would more effectively utilize the elevators already in place to cut down on average ride time. This proposal was still somewhat expensive, but much cheaper than the other two. The owners of the building were just about to hire the third firm when a fourth proposal was presented. After detailed review, the fourth proposal was immediately implemented. The fourth engineering firm suggested that full‐length mirrors be installed in every elevator. When the building residents were in front of a mirror, they fidgeted and adjusted their ties, checked their make‐up, and so forth and didn’t notice the length of the elevator ride. This proposal didn’t cost the owners very much at all and was dubbed a great success. The fourth company understood that the real problem wasn’t that the elevators were too slow, but that the residents thought the ride times were too long. Source: http://www.vexrobotics.com/ 4 1.4.2 Specification The design should begin with a specification (or design basis) defining the product, capacity, raw materials, utilities and site information. Other important information that needs to be specified is the degree of accuracy required, the time assigned for the project and any other constraints within which the design must be done. In addition to knowing the design specifications for the product and the availability of raw materials, the design can be controlled by items contributing to the annual operating factor. These include temperature of the cooling water, available steam pressures, fuel used, value of the by‐products, etc. If the project entails the design of an established process and product, a specification can usually be determined at the start of the project. For new products, the specification will have to be developed from laboratory data, pilot plant tests and product market research (Towler & Sinnott 2013). In many cases, the design problem is seldom fully specified. Therefore, assumptions are required to be made and can be refined later. 1.4.3 Conceptual design Once the design problem has been reasonably specified, the next phase of the design is generating various alternatives which meet the design objectives. There are numerous possibilities that can be generated and it is often impossible to enumerate all these alternatives (usually due to time constraints). Many different approaches are applied to generate alternatives. Traditionally, this involves adapting previously determined solutions for a similar process or brainstorming (El‐Halwagi 2006). A literature survey will often reveal general information and specific data important to the development of the design project. A good method of starting a literature survey is to consult recent publications on the subject matter. In this way, the design is based mainly on the experience of the designer. The experienced engineer usually prefers established methods, rather than unproven novel designs. This is because the time and cost involved in developing new processes is usually underestimated (Towler & Sinnott 2013). Advances in the process are made in small increments, thus reducing technological and financial risk. However, by relying on previous experience, innovation and new ideas may be limited and it may not be possible to determine the optimum solution. New approaches have been developed to generate and search among alternatives – this is termed process synthesis. Once the alternatives have been generated, these need to be analysed and evaluated. Each alternative is analysed in order to determine the performance of the system. The type of analysis carried out includes mass and energy balance. The results include a preliminary process flow diagram, including the material inputs and outputs of the process, process conditions (temperature, pressure, flow rates, compositions) and the energy consumption rates. The evaluation of each alternative would summarise all the main performance indicators in order to assess whether the objectives specified for the process have been met. The various alternatives can then be ranked according to their performance, and two or three alternatives which are deemed to be the most promising can be selected. 5 Project specification Conceptual design Initial evaluation. Process selection. Preliminary process flow diagrams. Basic process design Process flow diagram. Material and energy balances. Equipment specification and design. Material selection. Preliminary cost estimation. Detailed process design Piping and instrument diagram. Material and energy balances. Equipment design. More detailed cost estimation. Detailed engineering Mechanical design of equipment. Structural design and civil work. Piping design. Electrical design. Project cost estimation Purchasing/procurement Construction Commissioning Operation Figure 1.1: Phases of a chemical engineering design project 6 1.4.4 Basic process design Once the process has been selected, a more comprehensive analysis can be performed. This includes a detailed process flow diagram (PFD). The sequence of equipment and unit operations in the overall process are shown on the PFD. This allows the chemical engineer to simplify visualisation of the chemical process and indicate quantities of mass and energy transfer. A qualitative flow diagram indicates the flow materials, unit operations involved, equipment necessary as well as information on temperatures and pressures. A quantitative flow diagram shows the mass of materials required for the process. Figure 1.2 shows a qualitative PFD. You can also see a qualitative PFD on this website: https://justinust.wordpress.com/2016/05/08/d2‐establishing‐design‐criteria‐and‐ acceptability/ Figure 1.2: Qualitative flow diagram for processing jet fuel Source: https://commons.wikimedia.org/wiki/File:Engineering‐Chemical‐Process‐PFD‐Conventional‐ Merox‐Process‐Unit‐for‐Sweetening‐Jet‐Fuel‐or‐Kerosene.png Figures 2.7 – 2.9 in the prescribed textbook show a quantitative PFD. You can also see one on this website: https://justinust.files.wordpress.com/2016/05/quantitative‐process‐flow‐diagram‐srd.png Usually a computer‐aided process design program (or process simulator) is utilised to assist with the required design calculations such as mass and energy balances and energy requirements. 7 The specification of various types of equipment, such as vessels, exchangers and pumps, required in the process and their material of construction can also be determined. This will enable an estimate of the capital cost of process to be calculated. The quantity of utilities (steam, water, compressed air, electricity, etc.) required can also be determined, which will enable an estimate of the operating cost of the process. At this stage the decision will be made whether to continue with the project or not, based on the cost estimate. 1.4.5 Detailed process design As the project proceeds, more detail is included, in order to obtain a more definitive cost estimate of the project. The purchase cost estimate for each piece of equipment needs to be evaluated; required fixed capital investment is estimated from the total purchased equipment cost using the equipment cost‐ratio method. Operating labour and utilities requirements are determined, and a total cost estimate of the product could be calculated. Once the total product cost has been estimated, the design group is in a position to evaluate for management the attractiveness of the proposed process using measures of profitability such as rate of return, pay‐out time, or present worth. These methods are outlined in learning unit 6. Detailed specifications of equipment and instruments are determined. The piping and instrumentation diagram (P&ID), which shows valves, instruments and safety equipment, is developed. Once this is complete, the process engineer will work with other engineering disciplines, such as civil engineers for site preparation, mechanical engineers for design of vessels and structures, and electrical engineers for instrumentation and control. We will cover detailed design of heat exchangers, separation columns and pumps, compressors and expanders in learning units 3‐5. 1.4.6 Procurement, construction, commissioning and operation Once the detailed engineering has been finalised, the equipment can be purchased from various vendors and the plant can be constructed. Procurement and construction are usually carried out by an engineering, procurement and construction (EPC) contractor. Finally, when the construction is complete, the plant can be commissioned (start‐up). Commissioning entails getting the process to operate steadily. The design team may return to assist in start‐up and operation. 1.5 Impact of engineering design Environmental considerations need to be incorporated into engineering design in the same integral way that economic considerations have been implemented. It is important for the engineer to continually refine their designs in order to minimise use of materials, energy and labour. Designs should also be modified to fit into the environment harmoniously and with minimum disruption or degradation of natural ecosystems. Just as engineers apply safety factors in their design to compensate for uncertainties about the strength of their structures, they also need to consider and implement safety factors to compensate for uncertainties about the environmental consequences of their project. These changes imply the need to clearly define engineering philosophy and ethical 8 procedures and consequences regarding the impact of engineering design. Ethics and green engineering techniques should not be considered as an appendage to design, but rather as an integral part of it. It is important that safety and environmental considerations not be left until the final stage of the design project; rather these should be considered early on in the project in order to avoid the need for hazardous materials. Design for an inherently safe process and eliminate the need for elaborate safety controls and complex waste treatment systems. In learning unit 7 we will emphasise the engineer’s ability to ensure that the products and processes are safe, problem solve within an ethical framework and continue to consider new processes that are less harmful to the environment and pose less risk to society. Activity 1.1 1. 2. 3. 4. 5. 6. List the steps in the design process. At what stage of the design is a mass and energy balance introduced? What is the role of the PFD in the design? How does this assist the engineer? What is the difference between a PFD and a P&ID? What are the three principal types of flow sheets used in chemical process industries? To what extent are instruments and controls indicated in each of the three types of flow sheets? 7. This article by David Mody of Queens University provides an overview of chemical process design engineering: http://ojs.library.queensu.ca/index.php/PCEEA/article/download/3824/3876 Read through this article and construct a flow sheet of the steps in the design process. 1.6 Summary The aim of this learning unit was to introduce the key steps in the design process and their application in the design of chemical products and manufacturing processes. We gave you a brief introduction to design decisions that challenge product and process design teams. We mentioned the environmental impact of the design process to highlight the importance of green engineering and designing to account for inherent safety. Many of the topics introduced in this learning unit served to provide an overview of what we will discuss in the remainder of the learning units contributing to the design process. Sources consulted El‐Halwagi, MM. 2006. Process integration, volume 7. Amsterdam: Elsevier. Establishing design criteria and acceptability: Qualitative process flow diagram. (n.d.). https://justinust.files.wordpress.com/2016/05/qualitative‐process‐flow‐diagram‐srd.png [Accessed 21 September 2017]. Mody, D. (n.d.). An overview of chemical process design engineering. http://ojs.library.queensu.ca/index.php/PCEEA/article/download/3824/3876 [Accessed 24 September 2017]. 9 Process flow diagram: PFD conventional merox process unit for sweetening jet fuel. (n.d.). https://commons.wikimedia.org/wiki/File:Engineering‐Chemical‐Process‐PFD‐Conventional‐ Merox‐Process‐Unit‐for‐Sweetening‐Jet‐Fuel‐or‐Kerosene.png [Accessed 21 September 2017]. Towler, G. & Sinnott, RK. 2013. Chemical engineering design: principles, practice and economics of plant and process design. 2nd edition. Oxford: Butterworth‐Heinemann. Turton, R, Bailie, RC, Whiting, WB & Shaeiwitz, JA. 2009. Analysis, synthesis and design of chemical processes. 3rd edition. New Jersey: Prentice Hall. What is the engineering design process. (n.d.). http://curriculum.vexrobotics.com/curriculum/intro‐to‐engineering/what‐is‐the‐engineering‐ design‐process [Accessed 21 September 2017]. 10 Learning unit 2: Process flow sheet development and synthesis Reading: To complete this unit, study chapter 2 in your prescribed textbook, Chemical engineering design. 2.1 Introduction Have you ever wondered how a process flow sheet came about? What is the purpose and feasibility of a particular flow sheet? A flow sheet is a diagrammatic representation of equipment in the process. The development of a flow sheet could be described as a series of steps in which a conceptual process is iteratively evaluated, modified and re‐evaluated until it becomes practical. Preparation and presentation of the flow sheet are key in the process design. The flow sheet is used by a specialist design group to form the basis of their detailed design. Operating personnel would also use a flow sheet when preparing operating and training manuals. It is used to document a process, improve a process or model a new one. Process synthesis is the step in design where the chemical engineer selects the process units and decides how to interconnect these units to create a flow sheet. This unit covers the basics of process synthesis and flow sheet development and provides an overview of the steps involved in designing new products or processes. There are various very important decisions in the design process (such as the choice of which chemistry or reaction pathway will be used to formulate the desired product). Alternative design decisions need to be considered at the beginning of the conceptual design and subsequent optimisation is important to ensure a successful design. 2.2 Learning outcomes At the end of this learning unit, you should be able to construct an annotated PFD indicating various equipment, streams and utilities required explain the different types of flow sheet presentation discuss the importance of process synthesis and describe the various approaches to process synthesis apply the hierarchical approach to process synthesis to develop a flow sheet decide whether a process will be operating in batch or continuous mode identify the input‐output structure of a process define the recycle structure of the process and develop alternatives to the recycle structure utilise heuristics to synthesise a process 2.3 Flow sheet presentation Chemical engineers use flow diagrams to convey a considerable amount of technical information about processes. These diagrams present the flow of material, equipment and unit operations involved in the process. Flow diagrams provide a very effective channel of communication between the various people involved in the design and operation of a process. Besides providing qualitative information about the process, they can also provide quantitative information such as quantities and compositions of streams and process conditions (temperature, pressure and phase). 1 The main types of diagrams utilised by chemical engineers are process concept diagrams block flow diagrams PFDs piping and instrumentation diagrams The diagrams differ in level of detail and complexity. 2.3.1 Process concept diagram The first step in evaluating a process is to construct a process concept diagram. This would involve identifying the chemical reactions taking place, with the balanced chemical reactions forming a basis for the overall process concept diagram. Normally a single block is drawn to represent the concept of the process. Figure 2.1 shows a generic process concept diagram. The overall chemical reaction with the appropriate stoichiometry is normally written inside the block. The reactant chemicals are drawn as streams entering the process from the left, with the products leaving the process on the right. The number of streams would correspond to the number of reactants or products. Unwanted side reactions need to be considered as well, with the unwanted products leaving the process as a by‐product. Feed streams Process Product By‐products (a) Recycle Feed streams Process Purge Product By‐products (b) Figure 2.1: Process concept diagram demonstrating the input‐output structure (a – no recycle, b – with recycle and purge) When constructing the process concept diagram, ask the following questions (Douglas 1988): 1. 2. 3. 4. 5. 6. Should we purify the feed streams before they enter the process? Should the reversible by‐product be recycled or removed? Is a gas recycle and purge stream necessary? Is it viable to discard some of the unreacted reactants? How many product streams are required? What are the design variables for the input‐output structure and what are the economic trade‐offs associated with these design variables? 2 2.3.2 Block diagrams The block flow diagram is a pictorial representation consisting of rectangular blocks to represent either single equipment, groups of operations or a stage in the process, together with quantities and other pertinent properties of the main streams. The block flow diagram may be drawn for a single process, or alternatively may be drawn for a complete chemical complex involving many different chemical processes. The block flow diagram is useful as it provides an overview of a process, either at the beginning of process design for orientation purposes or later as a summary of the material balance of the process. It also provides a logical overview of the building blocks for most processes. An example of a block flow diagram for methanol synthesis from natural gas is given in figure 2.2. Reforming Cooling Desulphurisation Distillation Compression Methanol synthesis Methanol Figure 2.2: Block flow diagram for methanol synthesis from natural gas Example 2.1 Toluene (10 000 kg/hr) and hydrogen (820 kg/hr) are used as feedstocks for the production of benzene. The toluene and hydrogen are sent to a reactor where 75% conversion occurs, and the effluent is sent to a gas separator where the non‐condensable gases are discharged from the system. The bottoms of the separator provide a liquid feed to a still where the lighter benzene gas is collected as the distillate and the bottom toluene draw is recycled back into the reactor. Sketch a block flow diagram showing the reaction, the stream names and the mass flow of the inlets and outlets. 3 Mixed gas (2 610 kg/h) Hydrogen (820 kg/h) Reactor C7H8 + H2 C6H6 + CH4 Benzene (8 210 kg/h) Effluent Gas separator 75% Conversion of toluene Distillation column Toluene (10 000 kg/h) Mixed liquids Toluene recycle Benzene (8 210 kg/h) 2.3.3 Process flow diagram A PFD is more complex and contains significantly more information than a block flow diagram. The PFD contains most of the details that are critical for the design of the process. It provides information on equipment, material and energy balances required to understand the process. The diagram is also very useful during the operation of the plant, in order to train new operators/engineers and for troubleshooting the process. A typical PFD will contain the following essential information (Turton et al 2009): 1. The major pieces of equipment in the process will be represented on the diagram, along with a description of the equipment. Each piece of equipment will have assigned a number and a descriptive name. There is a certain convention used for numbering equipment, for example heat exchangers are assigned the letter E, reactors are assigned R. 2. All process flow streams will be included and identified by a number. A description of the process conditions (temperature, pressure, quantity or flow rate, phase and enthalpy) and chemical composition of each stream will be given. The data will be either displayed directly on the process flow diagram or included in an accompanying flow summary table. 3. All utility streams (steam, electricity, cooling water, etc.) supplied to major equipment will be shown. Each utility is also identified by letters, for example lps low pressure steam 3‐5 barg (sat) mps medium pressure steam 10‐15 barg (sat) hps high pressure steam 40‐50 barg (sat) htm heat transfer medium (organic): to 400 °C cw cooling water: from cooling tower 30 °C returned at less than 45 °C wr river water: from river 25 °C returned at less than 35 °C rw refrigerated water: in at 5 °C returned at less than 15 °C rb refrigerated brine: in at ‐45 °C returned at less than 0 °C cs chemical waste water with high COD ss sanitary waste water with high BOD 4 el ng fg fo fw electric energy (specify 220, 380, 440, 660V service) natural gas fuel gas fuel oil fire water Other features of the process flow diagram include the following: Equipment is represented symbolically (see figures 2.2 – 2.5 in the prescribed textbook). Lines with arrows represent the flow of material into and out of equipment. Generally materials enter from the left and leave from the right. Gas streams are usually at the top, whereas liquid and solid streams are towards the bottom. The PFD can be a very complex diagram, and care should be taken in its preparation. It should be clear and uncluttered, so that there is no miscommunication between the various people that will utilise the diagram. The PFD for the production of benzene is shown in figure 2.3. 5 Figure 2.3: PFD showing the hydrocracking process (Source: https://upload.wikimedia.org/wikipedia/fa/f/f3/Hydrocracking_process_flow_diagram.png) Activity 2.1 Sketch a process block diagram for a refinery stream containing a mixture of aromatics (this would include benzene, toluene, xylene and some of the heavier aromatics) extracted with a liquid solvent to recover the aromatics. Distillation is used to separate the solvent and aromatics and the solvent will be recycled to the extraction column. To separate the aromatics, three columns will be used to allow for the recovery of benzene, toluene and mixed xylenes. Share a copy of the sketch on the discussion forum with your peers. 2.3.4 Piping and instrumentation diagram The P&ID, also called the mechanical flow diagram (MFD), provides further details of the process that are essential for the construction and operation of the process. 6 The P&ID includes the arrangement of the process equipment, piping, pumps, instruments, valves and other fittings. The following information will be contained in a P&ID (Towler & Sinnott 2013): All process equipment, identified by an equipment number. The equipment should be drawn roughly in proportion and the location of nozzles shown. All pipes, identified by a line number. The pipe size and material of construction should be shown. The material may be included as part of the line identification number. All valves, control and block valves, with an identification number. The type and size should be given. Ancillary fittings that are part of the piping system, such as inline sight‐glasses, strainers and steam traps, with an identification number. Pumps, identified by a suitable code number. All control loops and instruments, with an identification number. Utility connections and lines. The information not included in the P&ID relates to operating conditions, flow quantities, pipe routing and support structures and foundations. Other engineering disciplines involved in the project will utilise the P&ID as the basis for the detailed design and construction of the process (Turton et al 2009). The mechanical and civil engineers will design and install equipment. Instrument engineers will identify, install and inspect control systems. Piping engineers will produce the plant layout and elevation drawings. Project engineers will develop the plant and construction schedules. Figure 1.7 on the website http://www.informit.com/articles/article.aspx?p=1915161&seqNum=3 shows a P&ID for distillation of benzene. Figure 5.22 in the prescribed textbook has a P&ID for an amine guard process unit. To refresh your memory on how to read a P&ID, see the clip from Technical Piping: https://www.youtube.com/watch?v=kv‐nHNa‐tYU A brief PowerPoint slideshow summarises the section covering block flow diagrams, PFDs and P&IDs: http://pselab.chem.polimi.it/wp‐content/uploads/2014/11/LAB7‐BFD‐PFD‐PID.pdf The KLM technology group also provides a free textbook explaining PFDs: http://www.klmtechgroup.com/PDF/EGD2/ENGINEERING_DESIGN_GUIDELINES_process_ flow_sheet_rev_web.pdf For a detailed explanation to understand why PFDs are so important, consult the following clip: https://www.youtube.com/watch?v=4IWoSPd6Xfk The following clips from LearnChemE may also be useful: https://www.youtube.com/watch?v=G1_iQtvepIg https://www.youtube.com/watch?v=Yx‐ SjGG1wHs&index=3&list=PL4xAk5aclnUjEuE_fvbyEts_oBpHYcwLY https://www.youtube.com/watch?v=1oZXwkddeZQ 7 2.4 Anatomy of a chemical manufacturing process In this section we will briefly consider how to choose between a batch and continuous process. We briefly discuss reactor yield by means of an example. We will introduce recycle structure of the process and evaluate the extent and ease of recycling. 2.4.1 Components of a chemical process Figure 2.4 provides a generic block flow diagram that shows a typical chemical process broken down into the different blocks. The six main blocks are reactor feed preparation reactor separator feed preparation separator recycle environmental control Recycle Raw materials Reactor feed preparation Reactor Separator feed preparation Separator Products and by‐products Waste streams Environmental controls Discharge to environment Figure 2.4: Generic block flow process diagram Let’s briefly discuss each of the six blocks: 1. The reactor feed preparation block is needed to change the process conditions of the feed streams as required by the process. For example, this would include pressure, temperature, purity and in some cases the phase of the feed streams as well. 2. All chemical reactions take place in the reactor block. Streams leaving this block contain the product, unused reactants as well as by‐products. 3. The separator feed preparation block prepares the outlet stream from the reactor, enabling conditions suitable for the separator in order to recover the product. 4. Separation of the product, unused reactants and by‐product as well as purifying of the product occurs in the separator block. This unit could involve distillation, absorption and extraction. 5. The recycle block represents the return of the unused reactants back to the reactor for further reaction. 8 6. The purpose of the environmental block is to reduce the waste emissions from the process so as to have minimal impact on the environment. 2.4.2 Batch or continuous process When comparing different processes, the advantages of continuous operation over batch operation must be considered. In a batch process a finite quantity of product is made within a specified period. This could be a matter of a few hours or even days. The batch process consists of feeding a fixed quantity of material into a vessel, followed by a series of unit operations. The unit operations could consist of mixing, heating, reaction, distillation, etc. and each unit operation takes place at scheduled intervals. At the end of the process, the product, by‐product and/or waste streams are removed and the equipment is then cleaned and prepared for the next process. In a continuous process feed is continuously sent to a series of equipment, and products, by‐ products and waste streams are removed continuously and are sent to storage or further processing if required. There are very few processes that use continuous operation only. Most processes are described as semi‐batch with part of the process occurring in batches, such as the off‐loading of materials. In many cases a continuous operating process is favoured as the costs are reduced by the use of automation and less labour. In addition to cost reduction, continuous operation favours increased productivity, reduces waste and improves quality by making it easier to spot and correct errors. For large‐scale operations, continuous operation has been found to be more economical, whereas batch operation is used where flexibility is required in production rate or product specification. When deciding between batch and continuous operation, there are a number of factors to consider. Some of the more important ones are listed in table 2.1 (Turton et al 2009). Table 2.1: Factors to consider when choosing between batch and continuous operation Factor Size Batch accountability/product quality Operation flexibility Advantage/disadvantage of batch Smaller throughput favours batch operation. As throughput increases, the size of equipment rapidly increases. When the product quality of each batch of material must be verified and certified, batch operation is preferred. For certain operations where the reworking of off‐spec product is not favoured, small batch operation is favoured. Batch operation allows for the same piece of equipment to be used for multi‐operations. For example, a stirred tank can be Advantage/disadvantage of continuous Economies of scale favour continuous operation for large throughput. Continuous or periodic testing of product quality is carried out, but potentially large quantities of off‐spec product could be produced. If schedule permits, the off‐spec product may be blended and reprocessed in a continuous process. Operational flexibility can be built into continuous processes but often leads to inefficient use of the capital. Equipment 9 used as a mixer, then a reactor, then as a stage of a mixer/settler for liquid‐liquid extraction. Standardised equipment ‐ multiple products Processing efficiency Factor Maintenance and labour Feedstock availability Product demand Often batch processes can be easily modified to produce several different products using essentially the same equipment. Examples of batch plants that can produce 100 different products are known. For such processes the optimal control and sequencing of operations are critical to the success of such a plant. Operation of batch processes requires strict schedule and control. Because different products are scheduled back to back, changes in schedules have a ripple effect and may cause an issue with product availability for customers. Separation and reuse of raw material is more difficult with batch processes. Advantage/disadvantage of batch Operating costs are higher as a result of equipment cleaning and preparation time. Batch operations are favoured when feedstock availability is limited. For example, canneries and wineries are batch processing facilities that often operate only part of the year. Seasonal demand for products such as fertilizers, gas‐line anti‐ freezers, de‐icing chips for roads and pavements, can easily be accommodated. Batch plants are flexible and can easily accommodate off‐ season products. not required for one process but needed for another may sit idle for a long time. Often continuous processes are designed to produce a fixed suite of products from a well‐ defined feed material. The product suite or slate produced from continuous processes is usually fixed. Equipment tends to be designed and optimised for a single or small number of operating conditions. Generally, as throughput increases, continuous processes become more efficient. For example, fugitive energy losses are reduced and equipment such as pumps, compressors etc. operate with higher efficiency. Recycling of unused reactants and the integration of energy within the process or plant are standard practice and relatively easy to achieve. Advantage/disadvantage of continuous Operating labour will be lower for continuous processes. Continuous plants tend to be large and operate throughout the year to be profitable. Difficult to accommodate seasonal products. However, similar but different products can be produced using the same process through a series of campaigns at different times of the year. Each campaign may last several months. 10 Rate of reaction to produce products Processes that involve very slow reactions and therefore require long residence times are favoured by batch operations. Equipment fouling When there is significant equipment fouling, batch operation is favoured as it is easier to carry out equipment cleaning because equipment cleaning falls under standard operating procedure. Safety Worker exposure to chemicals and operator error are higher. Controllability A problem may arise as the control used for equipment is complicated. This is as a result of the same piece of equipment being used for different unit operations. Very slow reactions require very large equipment. The flow through this equipment will be slow and dispersion could be a problem if a high conversion and/or plug flow is/are required. Significant fouling in continuous processes is a serious problem and difficult to handle. In some cases, operating equipment may have parallel lines to allow for continuous operation. These drive up costs, labour and safety issues. Safety records are generally good for continuous operations and risks associated with chemical exposure are eliminated. Easy to control. More research has been done on continuous processes. Activity 2.2 1. Most pharmaceutical companies use batch processing. Can you think of reasons for doing so? 2. In the following article the advantages and disadvantages of batch vs continuous production in the food industry are discussed: https://foodcrumbles.com/batch‐vs‐continuous‐scaling‐ up‐a‐food‐production‐process/ Briefly summarise the advantages and disadvantages of batch and continuous production in food processes. Which of the food products in your home do you think have been made using a batch process? 3. Tiger Brands is a major South African company involved in food manufacturing. Visit their website at http://www.tigerbrands.com/ and list which of the food products would use batch and continuous processes. 4. Unilever is another major company involved in both food processing as well as manufacturing home and personal care products such as Handy Andy and OMO. The following website has a brief overview of detergent manufacturing: http://www.cleaninginstitute.org/clean_living/soaps__detergents_manufacturing.aspx Would Handy Andy be made using a batch or continuous process? What about OMO washing powder? 11 2.4.3 Recycle structure of the process The raw material entering the process is extremely valuable for most processes and as a result accounts for 10‐50% of the operating costs, according to Peters and Timmerhaus (1991). However, Turton et al (2009) argue that this estimate is low and would be in the region of 75%. Douglas (1988) says that it would be 33–85%. From this we deduce that raw materials are extremely valuable and as a result we try to reuse and recycle the unused reactants. The extent of recycling depends largely on how easily the unused reactants can be separated from the product stream. This would depend on efficiency of raw material usage identification and definition of the recycle structure of the process issues affecting the recycle structure that lead to process alternatives 2.4.3.1 Efficiency of the raw material usage For most processes we find that the raw material is a large part of the operating cost. It is therefore important to evaluate the efficiency of the raw material usage. You first need to understand the difference between single pass conversion and the overall conversion in the process: Note that the single pass conversion considers the conversion over the reactor only, whereas the overall conversion will be over the entire system, including the recycle. The yield tells us what fraction of the reactant ends up in the desired product. Study examples 2.1 and 2.2 from the prescribed textbook. Study example 1.3 from the Pearson website: http://www.informit.com/articles/article.aspx?p=1314637&seqNum=2 2.4.3.2 Identification and definition of the recycle structure of the process How do we recycle the unreacted raw materials in a continuous process? It could be done in one of three ways: 1. Separate and purify the feed materials from the products first and then recycle. Over time, many different separation techniques have been developed to separate mixtures. Economic feasibility is the main factor when deciding whether to separate and which technique to use. Operating conditions such as temperature and pressure also need to be considered as well as the differences in chemical and physical properties. This would take into account differences in density, viscosity, etc. 12 Recycle Raw materials Reactor feed preparation Reactor Separator Products and by‐products 2. Recycle the product together with the feed materials without separating them and then using a purge stream (remind yourself what a purge stream is) to prevent build‐up. If separation between the feed materials and product is not easily achieved, we then consider recycling the mixture. When doing so, we consider whether the product would act as an inert or not, as well as equilibrium considerations and favouring of product. If the product does not react further, we would need to use a purge stream to prevent accumulation of the product within the process. Raw materials Reactor feed preparation Recycle Purge Reactor Products and by‐products 3. Recycle the feed and product together without using any purge stream. This scheme would only be feasible if the product reacts further when recycled to allow steady state operations to be achieved. Should this not be the case, then scenario 2 would have to be favoured. Recycle Raw materials Reactor feed preparation Reactor Products and by‐products Activity 2.3 1. Revisit example 2.1. Discuss which type of recycle is used. 2. The PFD for a hydrocracking process is shown in figure 2.3. Discuss the type of recycle used to recover hydrogen. 2.4.3.3 Issues affecting the recycle structure that lead to process alternatives 13 A number of issues contribute to the structure that determines where or how we place the recycle stream within the process. When deciding on the recycle structure, we consider the following (Turton et al 2009): How many potential recycle streams are there? Consider recycling reactants with a single pass conversion < 90%. How does the excess reactant affect recycle structure? Which reactant should be in excess and by how much? The recycle would then be proportionally larger. How many reactors are required? Reasons for multiple reactors are approach to equilibrium, temperature control, concentration control and optimisation of conditions to allow for multiple reactions. Do unreacted raw material streams need to be purified prior to recycling? Is recycling of an inert necessary? Can recycling an unwanted product or an inert shift the equilibrium to produce less of the unwanted product? In what phase is the recycle stream? This plays an important role in determining the separation and recycle structure of the process. For example, concerns about a liquid stream would be formation of an azeotrope that would complicate the separation scheme. For gases, concerns would be about temperature and pressure. 2.5 Synthesis of process flow sheets The first step in plant design is to define a process. A good starting point would be to define the chemical and physical processing steps to convert raw materials into desired products. There are a large number of choices and combinations of unit operations, reactor designs and ancillary processes that might be used, for even a simple process, and the task of making the correct choice can be quite daunting. As a result, chemical engineers have synthesised process flow sheets using experience, insight and mathematical programming. To adopt a systematic process in the synthesis of flow sheets, a hierarchy of steps for the designer to follow was developed. The purpose of a design hierarchy is to recognise that some steps in the design process precede others and guide the designer in making viable choices. Process synthesis hierarchies have been developed by Smith (2005) and Douglas (1988) and are described in detail in the prescribed textbook. Figure 2.5 shows a flow sheet adapted from Peters and Timmerhaus (1991) illustrating the hierarchy of the process when considering flow sheet synthesis and development. 14 Product definitions, information gathering, literature review Chemistry defined Preliminary economics No Economic potential? Identify process + sub-processes Define technology Equipment selection No Process evaluation - economics - safety Flow sheet Figure2.5: Flow diagram illustrating the hierarchal process in flow sheet synthesis and development We will briefly discuss process synthesis according to the Douglas approach in the following sub‐ section. 15 2.5.1 Process synthesis using the Douglas approach James M Douglas is credited with articulating one methodological approach to the design of chemical engineering systems, outlined in his textbook Conceptual design of chemical engineering processes. The Douglas approach is based on hierarchical decision‐making, using economic feasibility as a main criterion for process evaluation. The advantage of this is that design deficiencies are highlighted in the early stages of the process. The decisions are listed as follows: Process synthesis according to the Douglas approach 1. Decide whether the process will be batch or continuous. 2. Identify the necessary input and output structures of the process. 3. Identify the recycle structure of the process and develop the structure. 4. Identify and define the structure of the separation processes. 5. Identify and design the heat exchanger network or process energy recovery system. You begin by collecting the required information for the design. This includes physical properties, reaction information, production rate, raw material costs, site data and information regarding health, safety and environmental risks. Step 1: Decide whether the process will be batch or continuous ‐ see section 2.4.2. Step 2: Identify the necessary input and output structures of the process. To do this, you would need to evaluate whether the economic value of the reaction products exceeds that of the reactants. For synthesis to continue, the product value would need to exceed the reactant’s value. At this stage, conversion of the reactant has not been accounted for, nor have labour, overhead running costs, utilities etc. been taken into account. You need to take into account chemistry of the process and the extent of the reaction contributed to the overall input‐output structure. Step 3: Identify the recycle structure of the process. Take into account the extent of the different reactions occurring. Perform preliminary mass balance calculations. Plan to recover unreacted reactants and recycle to the reactor. Re‐evaluate costs of the raw materials and products. Step 4: Identify and define the structure of the separation processes. In step 3, we have assumed that separation may be perfect. Re‐evaluate based on separation principles. Product and by‐product specifications are considered. Determine actual separation compositions. Detail design is not included here. Step 5: Identify and design the heat exchanger network or process energy recovery system. Consider all heating and cooling mediums in the process. As supplying and removing heat is expensive, 16 consider heat and cool exchange between the streams. Carry out economic evaluation at this stage again. A summary of the proposed method is shown in figure 2.6. Figure 2.6: Conceptual design procedure proposed by Douglas (1988) 2.5.2 Heuristic rules in process synthesis When undertaking any sort of process synthesis logic, remember that there are certain rules of thumb in order to form a starting point for the design team. These rules of thumb or heuristics have been developed over a number of years and are often formed through the experience of the design engineer as well as texts they may consult. This would eliminate the need for extensive calculations. They may involve setting temperatures, pressures, excess amounts of chemicals, etc. The engineer should not shy away from using heuristics, nor should they rely blindly on them. Often we may find that one heuristic contradicts another and while it can reduce time in solving a problem, its acceptance will depend on the immediate context rather than taken as an absolute standard. Some of the heuristics as described by Seider et al (2006) are summarised here: Raw materials and chemical reactions o Select raw materials and chemical reactions to avoid or reduce the handling and storage of hazardous and toxic chemicals. 17 For competing series or parallel reactions, adjust temperature, pressure and catalyst to obtain high yields of the desired products. For an initial estimate, assume these conditions can be met. Obtain kinetic data and verify before making a base case. Separations o Attempt to condense vapour mixtures with cooling water. o Separate liquid mixtures using distillation, stripping, enhanced distillation, liquid‐ liquid extraction, crystallisation and absorption. Pumping and compression o To increase the pressure of a stream, pumping a liquid is preferred over compressing a gas (except in the case where refrigeration is required). o Use a fan to raise the gas pressure from atmospheric pressure to as high as 10.1 kPa gauge. Use a blower or compressor to raise the gas pressure to as high as 206 kPa gauge. Use a compressor or staged compressor to attain pressures greater than 206 kPa gauge. o A more extensive list of heuristics is provided in the following document: http://wp.auburn.edu/eden/wp‐content/uploads/2012/03/Heuristic‐Rules‐Table‐6.2‐from‐SSLW.pdf Now you can see that some of the heuristics provide guidance whereas others assist in designing the process and choosing equipment. It is therefore important for the designer to understand the context in which they will use the heuristics and to always re‐evaluate the validity of the heuristics in their particular situation. The following presentation describes the heuristics for process synthesis by means of examples: profsite.um.ac.ir/~fanaei/_private/Heuristics.ppt Example 2.2 has been adapted from Peters and Timmerhaus (1991) and illustrates the use of heuristics and process synthesis in flow sheet development. Example 2.2: Douglas approach as adapted from Peters and Timmerhaus (1991) Synthesis of a styrene process: Benzene is reacted with ethylene to form ethylbenzene. The ethylbenzene is then dehydrogenated to form styrene. This example explains the steps involved in synthesising a process to produce styrene from ethylbenzene. Heuristics are used as a guide to define the steps in the process. The overall reaction is as follows: ⇔ Costs of the chemicals are as follows: 18 Table 2.2 Chemical Styrene Ethylbenzene Hydrogen Steam Cost $0.42/lb $0.25/lb $0.30/lb $0.01/lb Step 1: Batch design Decide whether the process will be batch or continuous. Information based on the literature review (as the production capacity of the process is large) indicates that the styrene production from ethylbenzene would favour a continuous operation. Step 2: Input‐output structure of the process Determine the cost of producing the product, i.e. determine whether the value of the reaction products exceeds the value of the reactants. Table 2.3 summarises the cost of products and reactants. These were calculated based on the stoichiometry of the reaction and 1 lb‐mol of styrene product. Table 2.3 Chemical Styrene Hydrogen Ethylbenzene Cost products Cost reactants Cost/lb $0.42/lb $0.30/lb $0.25/lb Mass Cost 104 lb 104 0.42 $43.68 2 0.30 $0.60 2 lb 106 0.25 $26.50 106 lb 43.68 0.60 $44.28 $26.50 The product value exceeds the cost of the raw materials; therefore the process synthesis may continue. At this stage labour costs, equipment costs, overhead costs etc. have not been considered. The extent of the reaction has not been taken into account either. For this scenario, the cost as in table 2.3 is for the overall reaction. Let’s look at the costs involved if we consider the chemistry of the process thoroughly: Reaction (1) is an equilibrium reaction and does not go to completion. The reaction is in the gas phase over a solid, ferric‐oxide‐based catalyst. It occurs at low pressure, 1 atm, as the low pressure favours the products. Temperature of the reaction is at about 600 °C. Steam is added to lower the partial pressure of the products, thereby further favouring their formation. The reaction is endothermic, with the heat required being supplied by the steam. Steam to ethylbenzene molar ratio of 14:1 is required. ⇔ 1 The side reactions occur as follows: 19 ⇔ 2 And ⇔ 3 Reactions (2) and (3) consume ethylbenzene and thus lower the production of styrene. The products formed are styrene, hydrogen, benzene, ethylene, toluene, methane and condensed steam. Figure 2.7 summarises the input‐output structure of the process. Styrene product Ethylbenzene Process By‐products hydrogen benzene ethylene toluene methane Steam Condensed steam Figure 2.7: Input‐output structure of the styrene process The cost of product and reactants is summarised in table 2.4. Table 2.4 Chemical Styrene Hydrogen Ethylbenzene Steam Cost products Cost reactants Cost/lb $0.42/lb $0.30/lb $0.25/lb $0.01/lb Mass Cost 104 0.42 $43.68 104 lb 2 0.30 $0.60 2 lb 106 0.25 $26.50 106 lb 252 lb* 252 0.01 $2.52 43.68 0.60 $44.28 26.50 2.52 $29.02 Value of products still exceeds that of the reactants. *Steam required: Steam to ethylbenzene ratio is 14:1 therefore: 14 18 252 Step 3: Determine the recycle structure of the process. To do this, the extent of the different reactions is required. This would require reaction kinetic data. A detailed mass balance would then be carried out. Once the mass balance calculations are available, the cost of reactant and products is re‐evaluated. Figure 2.8 shows the proposed recycle structure should the reaction (1) not go to 20 completion. Table 2.5 summarises the mass balance and cost of the process with the recycle structure included. The mass balance is carried out with the following fractional extents: Reaction (1) extent = 0.47 Reaction (2) extent = 0.025 Reaction (3) extent = 0.005 Ethylbenzene Fuel gas Ethylbenzene Reactor Steam Condenser Separator Styrene Liquid fuel Water Figure 2.8: Recycle structure of the styrene process Table 2.5 Ethylbenzene Steam IN (lb/hr) 112.8 536 TOTAL 648.80 COST $/lb 0.25 0.01 Total Cost $ 28.2 5.36 33.56 Fuel gas Styrene Liquid fuel Water TOTAL OUT lb/hr 3.6 104 5.2 536 648.80 COST $/lb 0.18 0.42 0.10 No value Total Cost $ 0.65 43.68 0.52 44.85 Step 4: Separation processes From the recycle structure of the process, we have already ascertained that to recycle the unreacted ethylbenzene, some sort of separation is needed. In step 3, this is further broken down and the physical properties of the different components are considered. The hydrocarbon phase exiting from separator 2 needs to be separated into 3 fractions:1. benzene and toluene ethylbenzene styrene To do this, distillation will be done. An ordinary distillation column yields two products; therefore three distillation columns will be required to yield three hydrocarbons. First it is recommended that the lower boiling component (benzene and toluene) be removed. This is followed by ethylbenzene and the styrene‐rich stream (closest boiling points ‐ ethylbenzene and styrene). The desired product (styrene) is finally removed as the product in the third distillation column together with higher boiling “tars” that need to be removed. This process is depicted in figure 2.9. 21 Steam Reactor Ethylbenzene Condenser Fuel gas Water Separator 1 Separator 2 Liquid fuel Ethylbenzene recycle Distillation 1 Distillation 2 Distillation 3 Styrene product Tar Figure 2.9: Separation sequence for the styrene process Step 5: Heat integration Heating and cooling of materials are required in the styrene process. From past experience, we find that the reactor outlet temperature of 600 ˚C is required. The styrene reaction is operated without any heat added; the reaction itself is endothermic, causing the temperature to drop. To have the required temperature, the feed stream of ethylbenzene requires heating. The feed temperature is calculated from an energy balance around the reactor: ∆ ∆ ∆ Mass balance around the reactor: 112.8 An average 0.53 ° 122. 8 536 761.6 can be used as an estimate and ∆ / 56 000 / Express temperature in ˚F 1112 77 77 ∆ Solve for T = 1237 ˚F (670 ˚C) To maintain the reactor outlet temperature, an energy balance around the reactor shows that the feed of ethylbenzene requires heating to 670 ˚C. 22 For condensation of the reactor effluent (initially at 60 ˚C) to occur, the exit reactor effluent temperature of 40 ˚C is required. There is a possibility here that the feed stream to the reactor (which needs heating) could have a heat exchange scenario with the reactor effluent stream (which needs cooling). Heat integration between these two streams could be explored. Economic evaluation would be carried out again once the flow sheet has been finalised. Activity 2.4 1. 2. 3. 4. 5. 6. What are the principal types of flow sheets used in the process industry? Which type of flow sheet would you use to make a preliminary cost estimate? Summarise the five key points of the hierarchy of process design. Give three reasons why you would choose a batch process over a continuous process. List the recycle structures possible in a chemical process. Describe a scenario in which the feed would need processing before being fed to the process. 7. When would you add an inert material to the feed stream? What are the advantageous/disadvantages of doing so? 8. The method of preparing acetaldehyde is by the direct oxidation of ethylene. The process employs a catalytic solution of copper chloride containing small quantities of palladium chloride. The reactions may be summarised as follows: 2 2 2 2 1 2 2 →2 In the reaction, PdCl2, is reduced to elemental palladium and HCl, and is reoxidised by CuCl. During catalyst regeneration, the CuCl is reoxidised with oxygen. The reaction and regeneration steps can be conducted separately or together. In the process, 99.8% ethylene, 99.5% oxygen and recycle gas are directed to a vertical reactor and are contacted with the catalyst solution under slight pressure. The water evaporated during the reaction absorbs the exothermic heat evolved, and make‐up water is fed as necessary to maintain the catalytic solution concentration. The reacted gases are water‐scrubbed and the resulting acetaldehyde solution is fed to a distillation column. The tail gas from the scrubber is recycled to the reactor. Inerts are eliminated from the recycle gas in a bleed stream which flows to an auxiliary reactor for additional ethylene conversion. Prepare, in the form of a flow sheet, the sequence of steps in the development of a plant to produce acetaldehyde by this process. Include an analysis of the points to be considered at each step. List the additional information that will be needed to complete the preliminary design evaluation. 9. Consider the hydrodealkylation of toluene to benzene. The reaction is a high temperature and pressure reaction occurring at 35 bar and 620 ‐ 700 ˚C. The reaction is as follows: → A side reaction allowing for the formation of diphenyl occurs: 23 2 ⇔ Hydrogen in excess (5 to 1) is applied in order to avoid coke formation. The reactor product is also quenched at <620 ˚C. Pure toluene at atmospheric pressure is available as well as hydrogen gas (with 5% methane) at 38 bar. Using the Douglas approach, suggest and sketch the flow sheet path the process might take. 2.6 Summary In this learning unit, you learnt about the importance and development of a hierarchical approach to process synthesis. The first step was to determine whether the process will be batch or continuous by investigating the advantages and disadvantages of the two processes. We explored the construction of the process concept diagram, followed by the recycle structure. We presented heuristics and their application in relation to process synthesis of flow sheets. You discovered the types of flow sheets that the engineer will use in the design process. We explained process concept diagrams, together with the process block diagrams and process flow diagrams. Sources consulted Douglas, JM. 1988. Conceptual design of chemical processes. New York: McGraw‐Hill. Hydrocracking process flow diagram. (n.d.) https://upload.wikimedia.org/wikipedia/fa/f/f3/Hydrocracking_process_flow_diagram.png [Accessed 25 September 2017]. Peters, MS & Timmerhaus, KD. 1991. Plant design and economics for engineers. 4th edition. New York: McGraw‐Hill. Seider, WD, Seader, JD & Lewin, RL. 2006. Product and process design principles: synthesis, analyses and evaluation. 2nd edition. New Jersey: John Wiley and Sons. Smith, R. 2005. Chemical process design and integration. New Jersey: Wiley. Towler, G & Sinnott, RK. 2013. Chemical engineering design: principles, practice and economics of plant and process design. 2nd edition. Oxford: Butterworth‐Heinemann. Turton, R, Bailie, RC, Whiting, WB & Shaeiwitz, JA. 2009. Analysis, synthesis and design of chemical processes. 3rd edition. New Jersey: Prentice Hall. 24 Learning unit 3: Detailed heat exchanger design 3.1 Introduction In the process industries, properly designed equipment is essential for the production of on‐spec and cost‐effective products. The productivity, efficiency and safety of process plants hinge on proper equipment functionality and design. Heat transfer is a very important unit operation in chemical engineering and a considerable fraction of the capital cost of a chemical plant is invested in heat transfer equipment. The best known types of heat transfer equipment are heaters, coolers, condensers, boilers and furnaces. The thermal performance of various heat exchangers can vary quite a bit, so it is important to understand the process requirements as well as the different types of fluids available for removal of heat. It is also important to evaluate the entire system when deciding on a heat exchanger, as there are a number of considerations including flow rate, pressure drop, materials compatibility, and more. 3.2 Learning outcomes At the end of this learning unit, you should be able to describe the different types of heat exchangers discuss the advantages and disadvantages of using a shell and tube heat exchanger describe shell and tube construction details and layout explain general design considerations when designing a shell and tube heat exchanger undertake a thermal design of a shell and tube heat exchanger calculate pressure drop for a shell and tube arrangement discuss the different types of plate heat exchangers explain the flow arrangement for a plate heat exchanger undertake a thermal design of a plate heat exchanger calculate pressure drop for a plate configuration 3.3 Shell and tube heat exchanger design Reading: To complete this unit, study chapter 19 in your prescribed textbook, Chemical engineering design. A heat exchanger is used to transfer heat between two or more fluids. Although there are some heat exchangers where the two fluids are mixed directly (such as cooling towers), this is generally not the case and the two fluids are normally physically separated from each other. Heat is transferred by conduction through the exchanger materials which separate the material being used. The word "exchanger” applies to all types of equipment in which heat is exchanged, but is often used specifically to denote equipment in which heat is exchanged between two process streams. Exchangers in which a process fluid is heated or cooled by a plant service stream are referred to as heaters and coolers. If the process stream is vaporised completely, the exchanger is called a vaporiser, for a distillation column it is known as a reboiler and if it is used to concentrate a solution, then it is called an evaporator. The term “fired exchanger” is used for exchangers heated by combustion gases, such as boilers; other exchangers are referred to as "unfired exchangers". In the design of heat exchangers there are four major parameters: 1 Table 3.1: Parameters to consider when designing a heat exchanger Physical size The room available in a plant to locate a heat exchanger is often limited and then this parameter becomes important. Heat transfer required Every heat exchanger is designed to perform a specific heat duty. Pressure drop of fluid streams Every heat exchanger fits into one or more piping systems, so the pressure drops have an influence on the piping design. Heat exchanger cost The aim is to obtain an optimal economic design. 3.3.1 Classification of heat exchangers in terms of process function In this section we describe the most common types of heat exchangers used in the process industry. The following terms are generally used to describe the functions of heat exchangers and will be used throughout this unit: Table 3.2: Classification of heat exchanger in terms of process function Cooler Cools a process fluid by means of cooling water or air. Condenser Condenses a vapour or a mixture of vapours, either alone or in the presence of a non‐ condensable gas. Chiller Cools a fluid with a refrigerant (such as freon or ammonia) to a lower temperature than that obtainable when using cooling water. Exchanger Performs a double function by heating a cold fluid and cooling a hot fluid. Heater Adds sensible heat to a process fluid by means of hot utility such as condensing steam or thermal oil. Reboiler Provides heat necessary for distillation at the bottom of a distillation tower by means of a hot utility. Partial condenser Partially condenses a vapour or a mixture of vapours. Evaporator Concentrates a solution by the evaporation of water. Vaporiser A heater that vaporises any liquid (besides water). 2 3.3.2 Types of heat exchangers There are many different types of heat exchangers used in engineering practice. The geometry of the flow configuration, heat transfer surface and construction materials all depend on the design requirements. The most important geometric flow configurations are as follows: Counter current flow: In a counter current flow configuration, the fluids flow parallel to each other but in opposite directions. This type of arrangement is usually the most efficient as it allows for the largest change in temperature for both the fluids. Figure 3.1(A) shows the temperature profile for counter current flow in a heat exchanger. You can see a schematic (Figure 1) of counter flow heat exchange on the following website: http://www.thermopedia.com/content/832/. Figure 3.2 in this unit also shows counter current flow in a double pipe heat exchanger. Co‐current flow: In a concurrent flow configuration, fluids flow parallel to each other and in the same direction. A temperature profile for this type of a configuration is shown in figure 3.1(B). A co‐current configuration is not as efficient as a counter current configuration, but there is uniformity in the wall temperature. Figure 3.1: Temperature profiles for counter and co‐current heat exchange (Source: https://commons.wikimedia.org/wiki/File:Delta_T_1.png) 3.3.2.1 Concentric (double) pipe heat exchangers The simplest type of heat exchanger is the double pipe heat exchanger (figure 3.2). This type of heat exchanger basically consists of two concentric pipes. One fluid flows in the inner pipe and the other fluid flows in the annulus between the two pipes. Three or more different pipes can also be used in a similar arrangement (multitube units). 3 Figure 3.2: Double pipe heat exchanger (Source: https://commons.wikimedia.org/wiki/File:Double‐ Pipe_Heat_Exchanger.png) When arranged in two or more legs, the unit is called a hairpin. The greatest disadvantage of using a double pipe heat exchanger is the small amount of heat transfer area relative to the space required in comparison to other types of heat exchangers. They can be attractive for very high pressures because containment in small pipes is generally less costly. Commercially available double pipe heat exchangers use standard pipes from an internal diameter of 50 mm to 100 mm as outer pipes. The internal diameter of a pipe is measured at the mouth of the pipe and is referred to as the nominal bore (NB). Inner pipes are 25 NB to 80 NB with lengths up to 6 m. Heat transfer surfaces are normally smaller than 10 m2. In most cases the inner pipe has longitudinal fins on the outside of the inner pipe to extend the heat transfer surface. 3.3.2.2 Plate heat exchangers A plate heat exchanger consists of a stack of closely spaced metal plates, supported between top and bottom bars and held together in a frame (figure 3.3). Elastomeric gaskets separate the plates. At one end of the heat exchanger is a fixed head and at the other a movable follower. The whole pack is pressed together by the tie‐bars. Corner ports direct the fluid flow. Normally the gap between plates is between 3 mm and 6 mm. The plates have corrugation patterns to enhance heat transfer. The flow arrangement can be in series, in parallel or a combination of the two. Plate heat exchangers can be used for multiple duties; several different fluids can flow through different parts of the heat exchanger. Generally high pressure and temperature differences cannot be accommodated. Plate heat exchangers are generally used in the food and beverage industry, because it is relatively easy to take them apart for cleaning. They may also be used in the chemical industry, depending on customer requirements and standards. Plate heat exchangers are not suitable for condensation of large volumes. Although they are more compact and less expensive than shell and tube heat exchangers, they require more maintenance and therefore are not suitable for all applications. Standard plate heat exchangers can have a heat transfer area of up to 400 m2 with dimensions of 0.9 m (W) x 3.6 m (L) equipped with 400 plates. The following link provides a good visual simulation of a plate heat exchanger in industrial use: https://www.youtube.com/watch?v=Jv5p7o‐7Pms 4 We will discuss the thermal design of plate heat exchangers in further detail in section 3.4. Figure 3.3: Plate heat exchanger (Source: https://goo.gl/MwPPhs) 3.3.2.3 Air‐cooled heat exchangers Air‐cooled heat exchangers are used in operations where cooling water is scarce and in tropical climates (high temperature and high humidity) where it is economically viable and cheaper than cooling water. The following African countries, for example, have tropical climates and thus air‐cooled heat exchangers would be more viable: Democratic Republic of Congo, Zambia, Sudan, Angola and Central African Republic. In an air‐cooled heat exchanger, air is forced or induced by an axial flow fan over a bank of externally finned tubes. The fluid that needs to be cooled flows inside the tubes, and there can be one or more passes. At the tube ends, there can be a tube sheet with a header or U‐tubes can be used. The heated air is discharged into the atmosphere. Unit lengths can be up to 10 m with bundles of 4 m wide. Bundle depth can vary from 3 to 30 tubes. The most common tubes are from carbon steel with high‐fin‐type aluminium fins, but several types of finned tubes are commercially available. The sizes of the units can vary depending on the application. For example, a car radiator is very small in comparison to a vacuum steam condenser found on a power plant in the Medupi and Kusile power stations. It is interesting to note that these power stations have adopted dry cooling systems as a result of climate change. Read more about Eskom’s change from wet cooling systems to dry cooling systems by following these links: http://mydocs.epri.com/docs/SummerSeminar11/Presentations/04‐ 04_Lennon_Eskom_Dry_Cooling_v3.pdf and http://www.eskom.co.za/AboutElectricity/FactsFigures/Documents/CO0005CoolingTechniquesRev12.pdf The two main types of air‐cooled heat exchangers are either forced draft or induced draft heat exchangers. A forced draft heat exchanger has the fan located below the process bundle with air forced through the tubes. See figure 19.65 (b) in the prescribed textbook. An induced draft heat exchanger has the fan located above the process bundle and air is pulled through the tubes (figure 19.65 (a) in the prescribed textbook). 5 According to Perry and Green (2008), forced draft units use less power and have a longer mechanical life. Induced draft units induce a more even air distribution across the tube bundle. The higher outlet velocity of induced draft units prevents recirculation of already heated air. See how an air‐cooled heat exchanger operates by following this link: https://www.youtube.com/watch?v=3K9hBRWLb_M Read more about air‐cooled heat exchangers on the following website: https://www.aiche.org/resources/publications/cep/2017/january/improve‐air‐cooled‐heat‐exchanger‐performance 3.3.2.4 Shell and tube heat exchangers Shell and tube heat exchangers constitute the bulk of heat exchangers in chemical process plants. We will focus on this type of heat exchanger in this learning unit. In a shell and tube heat exchanger, one fluid is in a bundle of tubes and the other fluid to or from which heat is transferred is in a shell enveloping the tube bundle. The shell and tube heat exchanger consists of four major parts (see figure 3.4): Front header: This is where the fluid enters the tube side of the heat exchanger. Rear header: The fluid exits from the tubes at this end. Should there be multiple tube passes, it would return to the front header. Tube bundle: This consists of the tubes, tube sheets, tie rods, etc. that are holding the bundle together. Shell: This contains the tube bundle. There are various front header, rear header and shell designs. Before we study these, we discuss the standard used for shell and tube heat exchangers as these standards are used to classify the different types of headers and shells. Different standards have been developed for the design, fabrication, testing and designation of conventional shell and tube heat exchangers. Among others, the following standards are used: • TEMA Standards (Tubular Heat Exchanger Manufacturers Association (TEMA)) • British Standard, BS3274 • Heat Exchange Institute (HEI) Standards Throughout this unit we will use the TEMA standards. The TEMA standards use specific nomenclature for the various front headers, rear headers and shells, illustrated on the following links: http://www.engineeringpage.com/heat_exchangers/tema.html http://www.wermac.org/equipment/heatexchanger_part5.html Alternatively, see figure 19.9 of your prescribed textbook. As a result of the number of variations in mechanical designs for front and rear heads and shells, and for commercial reasons, TEMA has designated a system of notations that correspond to each major type of front head, shell style and rear head. The first letter identifies the front head, the second letter identifies the shell type and the third letter identifies the rear head type. For example, an AES heat exchanger implies that the heat exchanger consists of A: Channel and removable cover E: One‐pass shell S: Floating head with backing device 6 The principal types of heat exchangers are shown in figures 19.3 to 19.8 in the prescribed textbook. The following link gives a breakdown to the TEMA designations for the different heat exchangers: http://www.piping‐ designer.com/index.php/disciplines/mechanical/87‐stationary‐equipment/heat‐exchangers/2083‐shell‐and‐tube‐ heat‐exchanger‐tema‐designation Consider an AES shell and tube heat exchanger (see figure 19.6 in your prescribed textbook). The tube‐side fluid will enter the heat exchanger through one of the nozzles of the stationary head channel. The pass partition plate will prevent it from going out at the outlet nozzle and force it to flow through the tubes to the floating head. The fluid will then flow back through the tubes to the other side of the partition pass plate to the stationary head and out through the outlet nozzle. The shell‐side fluid will enter through the shell inlet nozzle and flow over the tubes to the shell outlet nozzle. The baffles ensure sufficient heat transfer (because of higher turbulence) and serve to support the tube bundle and to keep tube vibration within acceptable limits. The baffles are kept in place by a tie rod and spacer arrangement. Front header Rear header Figure 3.4: Shell and tube heat exchanger (Source:https://commons.wikimedia.org/wiki/File:Straight‐ tube_heat_exchanger_1‐pass.PNG) In terms of construction and mechanical cleaning, shell and tube heat exchangers can be divided into three categories: fixed tube sheet heat exchangers, U‐tube heat exchangers and floating head heat exchangers. Fixed tube sheet heat exchangers A fixed tube sheet heat exchanger has straight tubes that are welded to the tube sheet at both sides (figure 19.3 in your prescribed textbook). The tube sheet in turn is welded to the shell. You can see an example of a fixed tube heat exchanger by following this link: http://www.ansonindustry.com/pressure‐vessel/fixed‐tubesheet‐heat‐exchanger.html Or view the following link: https://www.youtube.com/watch?v=ioDspccjOhQ Normally a fixed tube sheet unit is the least expensive type of shell and tube heat exchanger. The tubes can be mechanically cleaned after removal of the channel cover or bonnet. The shell side, however, can only be chemically 7 cleaned. If the temperature differential between the tubes and the shell is large, an expansion joint will be needed because the tube sheets will be unable to absorb the differential stress without one. U‐tube heat exchangers In a U‐tube heat exchanger, the tubes are bent in the shape of a U. There will only be one tube sheet in a U‐tube exchanger. Figure 3.5 shows a U‐tube heat exchanger. In this case, there will be no problems with the expansion of the tubes. The tubes can easily be cleaned on the outside, but it is difficult to clean them on the inside near the bend. Figure 3.5: U‐tube heat exchanger (Source: https://commons.wikimedia.org/wiki/File:U‐tube_heat_exchanger.PNG) Floating head heat exchangers Floating head heat exchangers (see figures 19.6 and 19.7 in the prescribed textbook) are the most costly and most versatile type of shell and tube heat exchanger. Because the second tube sheet is free to float within the shell, the tube bundle can expand and differential temperature stresses can be avoided. The whole heat exchanger can be disassembled and mechanical cleaning can be carried out relatively easily on both the tube and the shell side. The following animation shows the construction of a floating head heat exchanger: https://www.youtube.com/watch?v=EMzne8d‐jJs Read more about a floating head heat exchanger at the following links: http://www.ansonindustry.com/pressure‐ vessel/floating‐head‐heat‐exchanger.html and http://www.thermopedia.com/content/1121/ Activity 3.1 Using the TEMA nomenclature, gives two examples of fixed tube‐heat exchangers U‐tube heat exchangers floating head heat exchangers 3.3.3 Basis of heat exchanger thermal design The general design equation for all heat exchangers is 8 Δ Although this equation is used for the design of all types of heat exchangers, our discussion will focus on shell and tube heat exchangers specifically. The symbol Q is used for the required duty of the heat exchanger (usually given in kW, BTU or kcal). This is the only symbol in the equation that cannot be manipulated and the required duty is basically a function of the specific process. Q will be equal to the heat removed from one fluid and will also be equal to the heat added to the other fluid. To refresh your memory (you should have covered this material in previous modules), consider the following example for the calculation of Q: Example 3.1 Saturated benzene at 101.3 kPa (abs) has to be condensed and sub‐cooled to 40 °C. The benzene flow rate is 4 000 kg/hr. Calculate the required duty. At 101.3 kPa (abs) the boiling point of benzene is 80.1 °C. The heat of vaporisation HVAP is 394 kJ/kg . The average heat capacity (cp) of benzene is 1.85 kJ/kg °C. The required duty (Q) will then be calculated as follows: ∙ 4 000 394 1576000 437,8 4 000 1.85 / 296740 80.1 40 / 82,4 520,2 Activity 3.2 Cooling water at a maximum flow rate of 20 000 kg/hr and a temperature of 23 °C is needed to condense and sub‐ cool the benzene in example 3.1. A counter current heat exchanger configuration is used where the water will be in contact with the cold benzene, at 40 °C. Calculate the following temperatures for the cooling water: a) The cooling water outlet temperature b) The intermediate cooling water temperature; include a temperature profile for the system (Answers: a) 45.3 °C ; b) 26.5 °C) The symbol A stands for the required heat transfer area (based on the outside tube diameter for shell and tube heat exchangers). When a heat exchanger is designed, we can rearrange the equation Δ To make A the subject of the formula: Δ 9 The most cost‐effective design will normally be the one that will have the smallest area and will still perform satisfactorily. Without knowing the meaning of and Δ at this stage, you should notice that numerically if the values of these symbols become larger, then the required heat transfer area gets smaller and the heat exchanger becomes cheaper. The overall heat transfer coefficient U can be defined as the reciprocal of the overall resistance ( transfer. The overall resistance to heat transfer is the sum of the individual resistances: 1 1 1 ∙ ln 2 / ∙ 1 ∙ ) to heat 1 Where: = outside film coefficient, W/m2 ˚C = inside film coefficient, W/m2 ˚C = outside dirt coefficient, W/m2 ˚C, table 19.2 in Chemical engineering design = inside dirt coefficient, W/m2 ˚C, table 19.2 in Chemical engineering design = thermal conductivity of the tube wall material, W/m2 ˚C, table 19.6 in Chemical engineering design = tube inside diameter, m = tube outside diameter, m The extent of the individual coefficients will depend on the nature of the heat transfer process the physical properties of the fluids the physical arrangement of the heat transfer surface This equation holds for liquids, gases, condensation, boiling, etc. You just have to make sure that you use the correct equation for the specific film coefficient. When designing a heat exchanger, choose an area that is larger than the required area as a safety factor. If the selected area is 10% larger than the required area, then the heat exchanger is 10% over‐surfaced. The outer diameter and standard lengths of commercially available heat exchanger tubes usually dictate the selected area. It is worth mentioning here that if your design tools are more accurate, you can live with a smaller percentage of excess area and the heat exchanger becomes cheaper. We will emphasise this point again at a later stage in this module. The mean temperature difference (Δ ) is an indication of the driving force for heat transfer. In most shell and tube exchanger configurations, there is a mixture of co‐counter, counter and cross‐flow current flow. To estimate the true temperature difference for counter current flow, a correction factor FT is applied. The correction factor depends on the number of shell and tube passes as well as the fluid temperatures. For shell and tube heat exchangers, the mean temperature difference can be calculated from the following equation: 10 Δ Δ Δ is called the logarithmic mean temperature difference and is defined as follows: ∆ Where: = hot fluid temperature, inlet = hot fluid temperature, outlet = cold fluid temperature, inlet = cold fluid temperature, outlet The mean temperature correction factor (Ft) is a dimensionless factor that has a value of 1 if the heat exchanger is of either true counter current or true co‐current configuration. If the number of passes in the shell and the number of passes in the tubes differ, the value of Ft has to be calculated. If one has multiple passes but the temperature of one of the fluids stays the same through the heat exchanger, then the value of Ft will also be 1 (for example condensing steam to heat a fluid). The correction factor is a function of the shell and fluid temperatures and the number of shell and tube passes. It is normally correlated from dimensionless temperature ratios: Figures 19.9 – 19.22 in the prescribed textbook provide correction factors for various shell and tube configurations. are as The assumptions made for the correction factor Ft in addition to the log mean temperature difference ∆ follows: 1. Heat transfer areas in each pass are equal. 2. There is a constant overall heat transfer coefficient for each pass. 3. Temperature in any shell‐side pass is constant across the section. 4. There is no leakage of fluids between shell passes. Note: An economical design cannot be normally achieved if the correction factor falls below 0.75. Example 3.2 illustrates the use of the logarithmic mean temperature difference. Use of the correction factor Ft will be illustrated in section 3.3.5, the worked examples. Example 3.2 The hot fluid enters a heat exchanger at 100 ˚C and exits at 90 ˚C, whereas the cold fluid enters at 30 ˚C and is heated to 50 ˚C. Calculate the log mean temperature difference for a) counter current configuration b) co‐current configuration 11 a) ∆ ∆ ∆ ∆ ∆ For a counter current heat exchanger ∆ 100 ∆ b) ∆ ∆ ∆ 50 50 and ∆ 50 60 54.9 50 ln 60 90 30 60 ∆ ∆ For co‐current flow: ∆ 100 30 70 ∆ and ∆ 70 40 70 ln 40 90 50 40 53.6 Notice that the log mean temperature difference is higher for the counter current configuration. What would this mean in terms of the area of the heat exchanger for the two configurations? Activity 3.3 Calculate Δ for the system described in example 3.1 with 30 000 kg/hr of cooling water at 23 °C for the following configuration: 1. A true counter current heat exchanger (one shell pass and one tube pass) 2. A true co‐current heat exchanger (one shell pass and one tube pass) (Note that because the benzene is first condensed and then sub‐cooled, you have to calculate a separate Δ for condensation and a separate one for the sub‐cooling. Which of the configurations will have the smallest heat transfer area and why? What will happen with the configurations in the activity if you use 20 000 kg/hr of cooling water at 23 °C?) 3.3.4 Shell and tube construction details 3.3.4.1 Tubes Tube diameters range from 5/8” (16 mm) to 2” (50 mm). Small diameters 5/8” to 1” are preferred as they give more compact (thus cheaper) heat exchangers. Larger tubes are easier to clean and are used for heavily fouling fluids. As a guide, ¾” (19 mm) is a good starting guess. Tube arrangements The tube pitch (shortest distance between two adjacent tubes) is normally 1.25 to 1.33 times the tube outer diameter (OD), but larger tube pitches are allowed. The tube orientation can be 30° (triangular), 60° (rotated triangular), 90° (square) or 45° (rotated square). Figure 3.6 in this unit shows the different tube layouts. Also see figure 19.10 in the prescribed textbook. If no mechanical cleaning on the shell side is required, it is customary to use a 1.25 OD triangular pitch. If mechanical cleaning is required, the best choice will usually be a 1.33 OD rotated square pitch. The tube pitch may sometimes be increased to reduce pressure drop, but designers usually prefer to use the smallest tube pitch possible in order to keep the shell size as small as possible. 45° or 90° layouts are chosen if mechanical cleaning is required, otherwise a 30° layout is often selected, because it provides a higher heat transfer and hence smaller exchanger. 12 Square Rotated Square Triangular 30˚ Triangular 60˚ Figure 3.6: Tube layouts (Source: https://commons.wikimedia.org/wiki/File:Uk%C5%82ad_rurek_w_p%C5%82aszczowo‐ rurowym_wymienniku_ciep%C5%82a.png ) Tube‐side passes Fluid in a tube is arranged in a number of passes to increase the length of the flow path. Figure 19.11 in the prescribed textbook shows tubes partitioned to give two, four and six tube passes. The number of passes is selected to obtain a reasonable tube‐side velocity. The bundle diameter therefore depends on the number of tubes (tube count) as well as tube passes. 3.3.4.2 Shells A shell diameter is selected to give as close a fit as possible to the bundle diameter, thus reducing bypassing of the fluid round the outside of the tube bundle. The TEMA standards cover heat exchangers up to 60 inches (1 524 mm) in diameter. Shell diameters up to approximately 24 inches (609.6 mm) are constructed from close tolerance pipe and above this value are rolled from plate. Shell arrangements are chosen depending on the pressure drop and heat transfer. The E‐type shell (a one‐pass shell ‐ see figure 19.9 in the prescribed textbook) is the most commonly used arrangement. For high pressure applications, the thickness of the shell is according to pressure vessel standards. A table of minimum shell thickness for various shell diameters is provided in section 19.5.3 in the prescribed textbook. 3.3.4.3 Tube sheet layout As part of the design process, the number of tubes to be used in the heat exchanger needs to be determined. The number and the length of the tubes create the area through which the heat is transferred from one process medium to another. This can be estimated as follows: 13 and / Where: = number of tubes = bundle diameter, mm = tube outside diameter, mm and = constants obtained from table 19.4 in the prescribed textbook The tube layout is normally planned with the aid of computer software. The following website has a tube sheet layout that you could use to test the accuracy of these empirical equations: https://red‐bag.com/tubesheet‐ layout.html 3.3.4.4 Baffles Baffles are used in the shell to direct fluid flow and increase the fluid velocity, thus improving rate of heat transfer. They are also designed to support tube bundles. Figure 19.13 in the prescribed textbook shows different baffle types. The segmental baffle is the most commonly used baffle. Baffle spacing is the centreline‐to‐centreline distance between adjacent baffles. Baffle spacing will usually vary between one‐fifth of the shell diameter and the shell diameter. The optimal baffle spacing will lead to the highest efficiency of pressure drop conversion to heat transfer. Sometimes the baffle spacing should be reduced to prevent possible vibration problems. To allow the fluid to flow backwards and forwards across the tubes, part of the baffle is cut away. The height of this part is referred to as the baffle cut and is measured as a percentage of the shell diameter. The range of the baffle cuts is between 15 and 45% and generally a baffle cut of 20 – 25% has been found to give optimum heat transfer. The size of the baffle cut needs to be considered along with the baffle pitch. It is normal to size the baffle cut and baffle pitch to approximately equalise the velocities through the baffle cut and in cross‐flow, respectively. 3.3.5 Individual film coefficients and pressure drop considerations 3.3.5.1 Tube‐side coefficients The physical properties that have the most pronounced effect on the tube‐side film coefficient are viscosity and thermal conductivity. The fluid properties are given in heat transfer applications and as they are inherent to the fluid, they cannot be manipulated. You can, however, get some advantage in terms of heat transfer if you put a highly viscous fluid on the shell side. In terms of tube‐side heat exchanger design, strive to get the velocity as high as possible. The velocity will be limited by the allowable pressure drop or, in a small number of cases, by erosion. The highest velocity possible will yield the highest film coefficient and the smallest amount of fouling. Assuming that the shell size is predetermined by shell‐ side conditions, the tube‐side velocity can be manipulated by changing 14 • • • the number of tube passes the tube size (tube diameter) the number of tubes Heat transfer data for turbulent flow is usually correlated from the equation: . . . Where: Pr = inside coefficient, W/m2 °C = equivalent or hydraulic mean diameter (= di for tubes), m = fluid thermal conductivity, W/m °C = mass flow per unit area kg/m2s = fluid viscosity Ns/m2 = fluid viscosity at the wall = heat capacity J/kg °C = 0.021 for gases = 0.023 for non‐viscous liquids = 0.027 for viscous liquids Heat transfer data for laminar flow is correlated from the equation: . . 1.86 . Where L = length of tube, m It is more convenient to correlate the heat transfer data in terms of a heat transfer factor . This enables data for both laminar and turbulent flow to be presented on the same graph, see figure 19.23 in the prescribed textbook. The heat transfer factor is defined by: / . Or also expressed in a more convenient form: 15 . . This does not adequately predict the coefficient for water, and a more correct equation was developed by Eagle and Ferguson (1930): 4 200 1.35 0.02 . . Where: = inside coefficient for water, W/m2 °C = water temperature, °C = water velocity, m/s = tube inside diameter, mm The two major sources of pressure loss are friction loss in the tubes and flow reversals that the fluid experiences through the tube arrangement. Figure 19.24 in the prescribed textbook gives the friction loss as a function of the Re number. The pressure loss due to friction (Pt) is then calculated from: ∆ 8 2.5 2 Where: ∆ = tube‐side pressure drop, N/m2 (Pa) Np = number of tube‐side passes ut = tube‐side velocity, m/s L = length of one tube jf = dimensionless friction factor, read off from the graph m = 0.25 for laminar flow, Re >2100 or m = 0.14 for turbulent flow, Re >2100 3.3.5.2 Shell‐side coefficients Before we assess the factors that have an influence on shell‐side heat transfer, you need to understand the flow phenomena in the shell side. Shell‐side flow can be divided into five streams (see figure 19.26 in the prescribed textbook or follow the link http://engrmaa.blogspot.co.za/2009_03_01_archive.html). The B stream is the most efficient in terms of heat transfer and the E stream the least efficient. The A, C and F streams also lead to partial heat transfer. A good design will attempt to get the highest part of the flow to be part of the B stream. Modern computer programs provide a flow (or stream) analysis to predict the flow fractions that will be allocated to the specific streams. The different types of shell patterns will also have different effects on the shell‐side film coefficients. According to some authors, the TEMA E (see figure 1 at http://www.globalspec.com/reference/46418/203279/chapter‐2‐heat‐ 16 exchangers or figure 19.9 in the prescribed textbook) dividing the shell into two parts. The F shell is sometimes useful if the tube‐side flow dictates two passes to get a true counter current arrangement. The TEMA G (split flow), TEMA H (double split flow), TEMA J (divided flow) and TEMA X (cross‐flow) are all TEMA shell types used for applications where the pressure drop should be very low (for example vacuum condensers) or sometimes to solve vibration problems. The TEMA K is used for kettle reboilers where they have a space for vapour disengagement. The shell‐side coefficient is difficult to predict due to the irregular flow pattern. Figure 19.29 in the prescribed textbook shows a curve for 2000<Re<106 and may be approximated by: . Shell‐side mass velocity and linear velocity Cross‐flow area Where: pt = tube pitch do = tube outside diameter Ds = shell inside diameter, m lB = baffle spacing, m For the equivalent diameter, it has been found that a better correlation is obtained using the mean hydraulic diameter along the tubes, rather than across. For square pitch arrangement: 4 4 1.27 0.785 For triangular pitch arrangement: 17 4 2 0.87 1 2 1.10 4 0.917 2 The shell‐side pressure drop is defined by the fluid flow across the bundles. Figure 29 gives the friction factor f for the shell side. The pressure drop for the shell is calculated: ∆ 8 . 2 Where: ∆ = shell‐side pressure drop, N/m2 (Pa) = number of tube‐side passes = shell‐side velocity, m/s =baffle spacing = tube length = dimensionless shell‐side friction factor, read off from the graph 3.3.6 General design considerations 3.3.6.1 Fluid allocation: shell or tube side Corrosion: The more corrosive fluid should be placed tube side. Fouling: The fluid that has the greatest tendency to foul should be placed tube side. This allows for easier cleaning. Fluid temperature: The hot fluid is placed in the tubes to reduce cost and prevent heat transfer loss. Viscosity: The more viscous fluid is placed shell side, provided the flow is turbulent. Higher heat transfer coefficients are obtained in this manner. Stream flow rates: Fluid with the lowest flow rate is normally assigned shell side for an economical design. 3.3.6.2 Shell and tube velocities High velocities reduce fouling but should not be so high as to cause erosion or too much of a pressure drop. The following serve as reasonable liquid and gas velocities: Liquid Tube: process fluids 1 to 2 m/s with a max of 4 m/s, water 1.5 to 2.5 m/s Shell: 0.3 to 1 m/s Vapour: velocity dependent on pressure Vacuum: 50 to 70 m/s 18 Atmospheric pressure: 10 to 30 m/s High pressure: 5 to 10 m/s 3.3.6.3 Stream temperatures The closer the approach temperature used, the larger the heat exchanger area required. As a general guide, the temperature difference should be at least 20 °C and the least temperature difference a minimum of 5 to 7 °C using cooling water. When the heat exchange is between process fluids, the approach temperature is not lower than 20 °C. 3.3.6.4 Pressure drop Suggested values for liquids Viscosity < 1 mNs/m2 Viscosity 35 kN/m2 1to 10 mNs/m2 50 – 70 kN/m2 Suggested values for gas and vapour High vacuum 0.4 – 0.8 kN/m2 Medium vacuum 0.1 × absolute pressure 1 to 2 bar 0.5 × system gauge pressure >10 bar 0.1 × system gauge pressure 3.3.7 Algorithm for design and rating procedures Before we define a general algorithm for the design of a shell and tube heat exchanger, it is important to note the difference between a rating problem and a design problem. The procedure for the two will be different. Rating a heat exchanger requires us to evaluate whether the given configuration available from a vendor fulfils the required load or not. Design, on the other hand, requires us to specify a configuration that will be suitable for a given service. Figures 3.7 and 3.8 show the algorithm for design and rating of shell and tube heat exchangers. The worked examples that follow will illustrate the use of the algorithm and applicable methods. 19 Figure 3.7: Heat exchanger design procedure Figure 3.8: Heat exchanger rating procedure 20 3.3.8 Worked examples Example 1 This example is adapted from the prescribed textbook, Chemical engineering design, section 19.9, example 19.1. The example as shown in the textbook is not very clear, so we will take a step‐by‐step approach and explain where everything comes from. The italic font indicates the logical steps that you have to follow and the normal font is the application to this specific example. Problem statement Design an exchanger to sub‐cool condensate from methanol condenser from 95 °C to 40 °C. Flow rate of methanol is 100 000 kg/h. Brackish water will be used as the coolant with a temperature rise from 25 °C to 40 °C. Solution This is an example of a design problem. Therefore, we apply the algorithm shown in figure 3.7. 1. Decide which fluid goes in the tubes and which in the shell. Normally water is allocated to the tubes. The following factors will determine the allocation: • • • • • • corrosion: more corrosive fluid in tube side fouling: liquid that is prone to fouling in tube side fluid temperature: the hotter fluid in tube side operating pressure: fluid with the lowest allowable viscosity: more viscous material shell side stream flow rates: lowest flow rate shell side allocated to tube side Coolant is corrosive, therefore it is assigned to the tube side. Water is in the tubes. 2. Check the heat load for both fluids to calculate the mass flow rate, m, or the missing temperature. 2.1 There is normally one unknown that has to be calculated. 2.2 The general equation for the heat load is: Δ Where: = = = = heat transferred per unit time, kW mass flow rate, kg/s heat capacity, kJ/kg°C difference in temperature in ( ) and temperature out ( ), °C 2.3 Use the bulk temperature to read off the value for if it is not given. 2 2 21 Where: = = = = cold fluid temperature in (= 25C) cold fluid temperature out (= 40 C) hot fluid temperature in (= 95 C) hot fluid temperature out (= 40 C) Δ Methanol: methanol = 2.84 kJ/kg °C (from tables of physical properties at bulk temperature. These would be found in handbooks such as Perry’s or CRC handbook of chemistry and physics. This type of information will be given to you in the exam). ∴ 100 000/3 600 2.84 95 40 4 340 4,2 / ° (from the physical properties table at bulk temperature in Perry’s or page 10, steam tables) Δ 4 340 68.9 4.2 40 25 ⁄ 3. Calculate the log mean temperature difference (°C) The basis for the calculation is counter current flow. For counter current flow the log mean temperature difference is given by: Where: = log mean temperature difference = inlet hot fluid temperature = outlet hot fluid temperature = inlet cold fluid temperature = outlet cold fluid temperature The golden rule to remember is that the capital letter T stands for the hot fluid side and the small letter t stands for the cold fluid side. The 1 always refers to incoming fluid and the 2 always refers to fluid leaving. For counter current flow the log mean temperature difference is given by: Δ ln 22 95 Δ Δ 40 95 40 40 40 25 55 15 ln 55/15 25 31° 4. Calculate ΔTLM and decide what type of heat exchanger you are going to use. The "true mean temperature difference" is determined by applying a temperature correction factor . is calculated by using: To get the value of we read it off figures 19.19 to 19.22 in Coulson and Richardson, volume 6. First calculate the factors R and S: And Choose 1 shell pass 2 tube pass exchanger. From: 95 40 40 25 3.67 40 95 25 25 0.21 From figure 19.19 in the prescribed textbook: 0.85 ∴Δ 0.85 31° 26° 5. Assume an overall heat transfer coefficient U for shell and tube heat exchanger. • • • Use table 19.1 and figure 19.1 in Chemical engineering design Units W/m2.°C Use any value between limits Choose U = 600 W/m2°C 6. Calculate the total heat transfer area A. 23 • • • Units: m2 Equation: Use Q and ° ∙ / ° Δ that you have calculated but use the value of U that you have chosen. 4 340 000 26° 600 / ° 278 7. Select/choose tube lengths and diameters. Calculate the number of tubes. If the tube outside diameter ( ) is assumed: specify from which table or standard • British Standard‐BS 3274 • American Standard ‐TEMA ‐ this you would find in older heat transfer textbooks, for example Kern (1950), table 10, appendix of calculation data. Towler and Sinnott (2013) recommend that 19 mm be used as a starting guess, as this is the most commonly used size in process industries. ranges from 6.35 mm to 63.5 mm. The larger tubes are for heavily fouling fluids. Select tube thickness • • • specify source base on pressure and corrosion allowance range 0.5 mm – 4.5 mm Select a preferred tube length (L) • • Page 1060 in Chemical engineering design 1.83 m, 2.44 m, 3.66 m, 4.88 m Allow for tube sheet thickness • • to 25 mm range it reduces effective length Use equation: • to calculate the area per tube. Remember to reduce the length to the effective length by 2 x tube sheet thickness. Calculate the number of tubes / Choose: 20 mm OD (outside diameter, do) 16 mm ID (inside diameter, di) 4.88 m long Allowing for tube sheet thickness: 4.88 4.83 2 25 24 20 10 0.303 4.83 / 278/0.303 928 8. Determine the type of heat exchanger construction (Towler and Sinnott, section 19.5) and calculate the shell diameter. • • • • • • internal floating head exchanger fixed tube sheet heat exchanger outside packed floating head exchanger U‐tube heat exchanger pull‐through floating head exchanger other heat exchangers 8.1 The shell diameter can be calculated: / For a 1 shell pass 2 tube pass exchanger and a triangular pitch, constants and Sinnott are: 0.249 and from table 19.4 in Towler 2.207 928 20 0.249 / . 829 Use a split ring floating head type: From figure 19.12 in Towler and Sinnott: Bundle clearance = 68 mm Therefore the shell diameter : 829 68 897 9. Tube‐side coefficient If there is water in the tubes, you can use a special equation for water: . 4 200 1.35 0.02 . 25 Where: = = = = inside coefficient (W/m2.°C) water temperature (°C) water velocity (m/s) tube inside diameter (mm) If it is any other fluid, use the following equation: . . Where: / and Calculate the velocity for water/fluid => Fluid mass velocity ( ) ‐ kg/s.m2 => Fluid linear velocity ( ) ‐ m/s [m2] Need: [kg/m3] look up in fluid property tables in Perry’s or steam tables Density of fluid / [kg/sm2] ⁄ ⁄ ⁄ Tube side velocity range: process: 1‐4 m/s water: 1.5 – 2.5 m/s Mean water temperature: = 40 25 ⁄2 Tube cross‐sectional area = ⁄4 Tubes per pass = ⁄2 Total flow area = 459 ⁄ ⁄4 16 918⁄2 201 Water mass velocity = 68.9 33 ° 459 (since we selected a 2 tube pass exchanger) 10 ⁄ 201 0.092 0.092 749 / Water density = 995 kg/m3 ∴ Water linear velocity = 749⁄995 ∴ 4 200 1.35 0.02 33 0.75 / – this is not within range of 1.5 to 2.5 m/s . . . 3 852 / ° 26 Compare the obtained using the special equation for water with the normal equation: . . Viscosity of water = 0.8 mNs/m2 Thermal conductivity = 0.59 W/m˚C 995 0.75 0.8 16 10 10 14 925 4.2 10 0.8 0.59 ˚ 10 ˚ 5.7 Determine • • First calculate / , where L = tube length and = tube diameter Use figure 19.23 in Towler and Sinnott to determine Viscosity correction factor • • If the fluid is not viscous, then ignore the viscosity factor of / If the fluid is viscous, then use the factor 483 10 16 3.9 From figure 19.23 in the prescribed textbook, 0.59 16 10 The 3.9 302 10 10 14 025 5.7 . 3812 / ˚ calculated using the two equations are reasonably close in value, therefore use the lower value. 10. Shell‐side coefficient Choose baffle spacing • • range: 0.2 – 1.0 shell diameters ( 1.25 ‐ mm pitch 0.2 0.2 894 1.25 20 ) 178 25 Calculate the cross‐flow area 25 20 25 894 178 10 27 0.032 Calculate the shell‐side mass velocity , and the linear velocity , / Where: = = fluid flow rate on shell side [kg/s] shell‐side fluid density [kg/m3] Shell‐side velocity range: 0.3 to 1 m/s 100 000 3 600 Methanol density 750 868⁄750 1.157 ∴ 1 0.032 868 / / / – This is high for the shell side where normal range is 0.3 to 1 m/s Calculate the shell‐side equivalent diameter Square pitch: . Triangular pitch: 0.785 . . Choose triangular pitch: 1.10 25 20 0.917 20 14.4 Calculate the shell‐side Reynolds number To calculate the Reynolds number we need the physical properties at the bulk temperature. Mean shell‐side temperature: (95 + 40)/2 = 68 °C From the physical properties table: • • • Viscosity methanol = 0.34 mNs/m2 Heat capacity = 2.84 kJ/kg °C Thermal conductivity = 0.19 W/m °C ∴ 868 14.4 10 36762 Determine For the selected baffle cut and the value of Reynolds calculated above, read off the value of in Towler and Sinnott. in figure 19.29 28 Choose 25% baffle cut 3.3 From figure 19.29 10 Calculate the shell‐side coefficient ⁄ 2.84 . . 3.3 10 10 36762 5.1 0.34 0.19 . . ⁄ ˚ 10 ˚ 2 740 / 0.51 °C Viscosity correction term is ignored as fluids are low‐viscosity fluids. Overall coefficient U To calculate the overall coefficient you need: (thermal conductivity of tube wall material) W/m °C table 19.6 Chemical engineering design (outside dirt coefficient) W/m2 °C table 19.2 Chemical engineering design (inside dirt coefficient) W/m2 °C table 19.2 Chemical engineering design Units of U W/m2 °C 1 1 ln 1 2 If U is not close to the assumed value of U, start the iteration using the calculated value of U as the next value of U. The kw value of the tubes assuming it is made from cupro‐nickel is 50 W/m °C. Take both fouling coefficients as 6 000 W/m2 °C. Then: 1 1 2740 1 6 000 20 10 ln 2 50 885 20 16 ⁄ 20 16 6 000 20 16 3 616 ° This is above the chosen value of 600 W/m2 °C, thus we have over‐designed the heat exchanger. 11. Calculate pressure drops Tube side: For the tube side From figure 19.24 14 925 4.3 10 Viscosity is low, therefore ignore the viscosity correction factor. 29 ∆ 2 8 ∆ 2.5 8 4.3 4.83 10 16 10 7211 ∆ 2 2.5 995 0.75 2 7.2 Pressure drop is low; increasing the number of tube passes could increase the pressure drop. Shell side: 868 750 4 From figure 19.30 in the prescribed textbook ∆ 8 4 10 1.16 36 762 for . ∆ 8 10 894 14.4 2 ∆ 750 4.83 10 178 272 019 1.16 2 272 Shell‐side pressure drop is too high. Increase baffle pitch: doubling the pitch would halve the shell‐side . velocity and reduce pressure drop by ∆ This is reasonable. Recalculate 272 4 68 : 2740 1 2 . 1573 / ˚ Recalculate U = 615 W/m ˚C. This is acceptable as it is above 600 W/m ˚C. Example 2 This problem is an example of a rating for an existing heat exchanger. We will apply the algorithm shown in figure 3.8. The vapour pressure of high‐octane motor fuel (70 m3/h) is such that it can be stored at a maximum temperature of 38 °C. The motor fuel leaves optimisation heat exchangers at a temperature of 91 °C. Limited cooling water (tube‐ side fluid) is available at 27 °C and may not be heated above 43 °C. Evaluate the given heat exchanger for this service and calculate the pressure drop limits. 30 PHYSICAL PROPERTIES Fuel Water units ρ 787 998 kg/m3 µ 0.28 1 cP Cp 2.07 4.19 kJ/kg ˚C kf 0.523 ‐ kJ/h m ˚C hid and hod 22 000 18 000 kJ/h m2 HEAT EXCHANGER CONFIGURATION Shell inside diameter Tube configuration Tube outside diameter Tube inside diameter Tube arrangement Number of tubes Tube length k(wall) Baffle spacing Baffle cut 387 mm 2 tube passes 19.05 mm 14.83 mm 15/16" (inches) triangular pitch 192 6 044 mm (tube sheets excluded) 52 W/m C 350 mm 25 % Solution 1. Calculate the heat flow Δ 70 787 2 070 3 600 1 678 800 / 1 678.8 91 38 ° ° Did you notice how flow rate in m3/h was converted to the mass flow rate of kg/s? 2. Determine missing flow rate Δ of the water (the cp of water is 4.19 kJ/kg °C) 1 678.8 10 4 190 25.04 ° 43 20 ° / 31 3. Calculate 91 43 38 ln 91 43 / 38 Δ 27 27 25.1 ° 4. Calculate the factors R and S We know the heat exchanger is a 1 shell pass 2 tube pass exchanger. From: 91 43 38 27 3.3 43 91 27 27 0.25 From figure 19.19 in Chemical engineering design 0.84 You would notice that the value of Ft is below 0.75 0.84 ∴Δ 25.1° 21.1° 5. Determine the heat exchanger area available Since this is a rating problem, there is no need to assume a U value. We can calculate the area available for the given configuration. 192 19.05 10 6.044 69.45 In this case we ignored the tube sheet thickness. But Δ ° / ° The A is known because the dimensions of the heat exchanger are fixed as well as the Q and Δ unknown is the overall coefficient. ∴ ⁄ Δ 1 678 800 69.45 21.1 . The only ° 32 ⁄ 1 145.6 ° 6. Tube‐side coefficient There is water in the tubes so we can use the special equation for water! Remember that you can also use the normal equation even though it is water in the tubes, but you will save a lot of time if you use this special formula for water because you don't have to look up any physical properties for water. 4 200 1.35 . 0.02 / . Where: = = = = 43 27 inside coefficient (W/m2 °C) water temperature (°C) water velocity (m/s) tube inside diameter (mm) ⁄2 35° 6.1 Calculate the velocity for fluid /4 ⁄4 14.83 172.7 /2 192/2 96 96 172.7 0.01658 10 25.04 / 1 510.25 / 998 0.01658 / ⁄ ⁄ 1 510.25 998 1.513 / ∴ Which is in the range of 1.5 – 2.5 m/s for cooling water 6.2 Calculate the coefficient ∴ 4 200 1.35 6 993 0.02 35 . . /14.83 . ° 7. Shell‐side coefficient 7.1 Baffle spacing is given as 350 mm 350 Pitch is also given as 15/16 inches. We need to convert this value to mm. 33 15 16 ∙ 23.44 25.4 triangular 7.2 Calculate the cross‐flow area 23.44 19.05 0.387 0.350 23.44 0.02537 7.3 Calculate the shell‐side mass velocity and the linear velocity fluid flow rate on the shell side [m3/s] ⁄ 70⁄3600 0.718 0.02537 / The range for the velocity in the shell is between 0.3 and 1 m/s, so the velocity is within the range. 7.4 Calculate the shell‐side equivalent diameter 1.10/ Triangular pitch: 1.10/19.05 23.44 12.48 0.01248 0.917 0.917 19.05 7.5 Calculate the shell‐side Reynolds number 2 070 ° 0.28 0.523 787 / ° / ∴ 0.718 12.48 10 0.28 10 787 25 185 7.6 Determine Baffle cut = 25% given From figure 19.29 in Chemical engineering design 3.8 10 34 7.7 Calculate the shell‐side coefficient ⁄ . ⁄ We can ignore the viscosity term because fuel is not a viscous fluid, i.e. ⁄ 1. / Now we must be careful of the units. The Prandtl number is dimensionless and the physical data given for , is not in SI units. The units for , are given in (kJ/h m °C) instead of (W/m °C). Thus we have to compensate for the difference in units! ⁄ ⁄ ° ⁄ 3 600 ⁄ ° Check yourself that the Prandtl number is dimensionless. 2.07 3 600 0.28 10 0.523 3.99 0.523 3.8 10 0.01248 ° 6 361.2 / 1 767 / ° 25 185 3.99 ˚ 8. Overall coefficient 1 1 ln 1 2 Assume: value of the tubes made from commercial steel is 52 W/m °C. The fouling coefficient units are given The in kJ/h m °C. We must first convert it to W/m °C before we can calculate U. 22 000 ⁄ ° 18 000 ⁄ ° 22 000/3 600 / 18 000⁄3 600 ° / 6 111 ° / 5 000 ° / ° Then: 1 1 1 1 1 767 1 5 000 0.000566 0.0002 10 ln 2 52 0.0000459 19.05 14.83 19.05 14.83 6111 0.000210 19.05 14.83 6 993 0.000184 0.00120583 829.3 But 19.05 / ° Δ 35 ∴ 1 678 800 829.3 21.1 Δ 95.94 And area which is available is 69.45 m2. Thus the area available is smaller than the area needed. The heat exchanger would not be suitable for this application. 9. Calculate pressure drops Tube side . For the tube side . 22 392 / 4.0 From figure 19.24 10 Viscosity is low, therefore neglect the viscosity correction factor. 2 8 ∆ 2.5 8 ∆ 4.0 10 6044 14.83 2.5 35 502 ∆ 2 998 1.513 2 35.5 For tube side, typical pressure drop is 30 – 60 kPa, therefore pressure drop is reasonable. Shell side From figure 19.30 in the prescribed textbook at ∆ ∆ 8 4.2 2 387 12.48 ∆ 10 . 8 10 4.2 25 185 , 36499 6.044 0.35 787 0.718 2 36.5 For the shell side, pressure drop is typically between 20 – 30 kPa; hence this is slightly high in this particular case. You could watch an additional example here: https://www.youtube.com/watch?v=NherLU3IVPY Activity 3.4 1. 75 tons per hour of kerosene will be heated from 25 ˚C to 50 ˚C by cooling a gasoline stream from 70 ˚C to 50 ˚C. Inlet pressure will be 3.5 bar for each stream and the maximum pressure drop of 0.5 bar for gasoline and 0.7 bar for kerosene are permissible. Published fouling factors for oil refinery streams 36 should be used for this application. Design a shell and tube heat exchanger for this service. (Answer: A= 200 m2, heat exchanger 1 shell with 6 tube passes, 1” tube OD and 1.25” sq. pitch) 2. Design a shell and tube heat exchanger to cool demineralised water, initially at 93 ˚C and at a rate of 6 840 kg/hr. Clean water flowing at 35 kg/s is available for this purpose. The initial temperature of the cooling water is 38 ˚C and the outlet of water should not exceed 54 ˚C. The velocity in the tubes should be 0.5 m/s. The heat exchanger should fit horizontally in a room 5 m by 5 m. The tubes must be removed periodically for inspection. Calculate the length of the tubes as well as the number of tube passes and the total number of tubes for the exchanger. (Answer: L = 2.4, number of tubes = 72, 4 tube passes) 3. For an acid catalysed hydration reaction, iso‐butene needs to be heated from 20 ˚C to 35 ˚C before entering a reactor. Dirty plant water enters the exchanger at 90 ˚C at a flow rate of 48 kg/s. The outlet temperature of the water is 80 ˚C. The tubes of the heat exchanger must be removable for cleaning purposes. The following unused heat exchanger is available: Tube length 3.66 m Tube OD 25.4 mm steel Tube ID 22.098 mm No. of tubes 166 No. of tube passes 2 Square pitch 1.25 OD Shell ID 540 mm No. of shell passes 1 Baffle spacing 729 mm Baffle cut 25% Tube sheet thickness 0.025 m ∆ < 60 kPa ∆ < 30 kPa Will this exchanger meet the requirements for the process? (Answer: Area available = 48 m2) 4. A shell and tube heat exchanger calculator is available on the following website: http://chemicalengineeringnow.com/HeatExchanger.aspx Watch the video to see how to input variables into the calculator here: http://chemicalengineeringnow.com/ShellandTubeVideo.htm Using the calculator, redo examples 1 and 2. What can you say about your answers? 37 3.4 Plate heat exchanger design A plate heat exchanger consists of a number of plates with ports allowing for the passage of two fluids to flow through. In this manner heat transfer between the fluids can take place. Normally, the plate pack is assembled between a fixed plate and a movable pressure plate. The number of plates is determined by the flow rate, the physical properties of the fluids, pressure drop and process temperature. Plate corrugations promote fluid turbulence and support the plates against differential pressure. The plate and the pressure plate are suspended from an upper carrying bar and located by a lower guiding bar, both of which are fixed to a support column. Connections are located in the frame plate to allow for either one or both fluids to make more than a single pass. We will briefly study the different types of plate heat exchangers in the following section, and discuss thermal design of a gasketed plate heat exchanger. The advantages and disadvantages of using a plate heat exchanger are summarised in table 3.3. Table 3.3: Advantages and disadvantages of a plate heat exchanger Advantages Disadvantages Economically viable Cannot accommodate high pressures > 30 bar Easy to maintain Gasket selection is critical Caters for low approach temperatures Cannot accommodate high temperatures > 250 ˚C Allows for dirty/fouling process mediums Difficult to clean clogged, narrow pathways Suitable for highly viscous liquids High heat transfer in gas treatment 3.4.1 Types of plate exchangers Reading: To complete this unit, study chapter 19.12 in your prescribed textbook, Chemical engineering design. 3.4.1.1 Gasketed plate heat exchangers A gasketed plate heat exchanger consists of closely spaced plates stacked together between a fixed frame plate and a movable pressure plate. The plates are normally between 0.5 and 3 mm thick with a gap between them of 1.5 to 5 mm, depending on the process application. You can see the layout of a gasketed plate heat exchanger in the prescribed textbook, figure 19.56. You could also view the specifications and materials of construction for gasketed plate heat exchangers on the following website: http://www.apiheattransfer.com/Product/37/Gasketed‐Plate‐Heat‐ Exchangers The following clip explains the operation of an industrial gasketed plate exchanger: https://www.youtube.com/watch?v=rD3badrHQrk 3.4.1.2 Welded plate exchangers A welded plate exchanger is very similar to the gasketed plate exchanger. The main difference is that the edges are sealed by welding rather than gaskets. This allows for higher temperature and pressure operation as well as avoiding leakage. The following clip from the manufacturer for a welded plate exchanger is useful in understanding the construction of such a heat exchanger: https://www.youtube.com/watch?v=sf4gkCNSILA 3.4.1.3 Plate‐fin A plate‐fin heat exchanger uses plates and fins to effect heat transfer between fluids. Figure 3.9 shows the components of a plate‐fin heat exchanger. In a plate‐fin exchanger, corrugated metal is placed between two flat 38 sheets (parting sheet) and held together by the side bars. Heat is transferred from one stream through the fin interface to the separator plate and through the next set of fins into the adjacent fluid. The fins have the dual purpose of holding the plates together, thus containing pressure, and of forming a secondary surface allowing for heat transfer. Figure 3.9: Components of a plate‐fin heat exchanger (Source: https://en.wikipedia.org/wiki/File:Principal_Components_of_a_Plate_Fin_Heat_Exchanger.jpg) 3.4.1.4 Spiral heat exchangers These types of heat exchangers are very similar to plate exchangers with the plates formed into spirals. In such a configuration, fluid flow may be counter, co‐counter or cross‐flow as in a shell and tube design. The main advantage of a spiral heat exchanger is the compact design and self‐cleaning mechanism that allows for use of dirty and fouling process duties. They perform well in applications such as pasteurisation, heat recovery, pre‐heating and effluent cooling. Figure 3.10 shows a schematic of a spiral heat exchanger. View an animation of a spiral exchanger operation by following the link: https://www.youtube.com/watch?v=83cNchNCh5U 39 Figure 3.10: A spiral heat exchanger (Source: https://en.wikipedia.org/wiki/File:Spiral‐heat‐exchanger‐schematic‐ workaround.svg) 3.4.2 Plate heat exchanger design In the following section we briefly discuss plate heat exchanger design. The design algorithm is very similar to that of a shell and tube heat exchanger. It will not be possible to give the exact design methods of plate heat exchangers as these largely rely on consultation with manufacturers and vendor‐specific methods. 3.4.2.1 Flow arrangement As with a shell and tube heat exchanger, flow configurations are either co‐current, counter current or cross‐flow arrangement. The streams can also be divided into number of passes and more plates could be added to increase the heat transfer area. See figure 19.17 in the prescribed textbook. 3.4.2.2 Temperature correction factor The temperature correction factor, is expressed in terms of the number of transfer units (NTU) and depends on the flow arrangement. The NTU is given by: ∆ Where: = stream outlet temperature, ˚C = stream inlet temperature, ˚C ∆ = log mean temperature difference, ˚C The temperature correction factor is then determined from figure 19.58 in the prescribed textbook. For most applications the NTU is between 2.0 and 3.0 with a range from 0.5 to 4.0. 3.4.2.3 Heat transfer coefficient The heat transfer coefficient can be calculated for turbulent flow: 0.26 . . . Where: = plate film coefficient, W/m2 °C = equivalent or hydraulic mean diameter, taken as twice the gap between the plates, m = fluid thermal conductivity, W/m °C 40 = mass flow per unit cross‐sectional area = , kg/m2s = mass flow rate per channel, kg/s = cross‐sectional area for flow, m2 = channel velocity, m/s = fluid viscosity Ns/m2 = fluid viscosity at the wall There is no heat transfer occurring at the end plates; thus the total number of plates is total number of plates less two. 3.4.2.4 Pressure drop The pressure drop equation used is the same as the tube‐side pressure drop calculation for isothermal flow in the tubes and takes into account the pressure drop due to expansion and contraction through the ports in the plates: ∆ 8 2 ∆ Where: = the path length = ∆ = pressure drop due to expansion and contraction losses in ports = 1.3 = velocity through the ports = area of the port , m/s , m2 = port diameter, m = number of passes 3.4.3 Algorithm for plate exchanger design The design procedure for a plate exchanger design is similar to that of a shell and tube heat exchanger. Figure 3.11 summarises the procedure. 41 Figure 3.11: Plate exchanger design procedure Study example 19.13 in the prescribed textbook. Activity 3.5 1. Discuss the advantages and disadvantages of using a plate heat exchanger. 2. Do problem 19.12 in the prescribed textbook, Chemical engineering design. 3.5 Summary Having completed this learning unit, you should be familiar with the different types of heat exchangers generally used in the industry. We discussed shell and tube construction details and layout and the advantages of a shell and tube heat exchanger. We explained general considerations for the design of a shell and tube heat exchanger and explored the thermal design of both plate and shell and tube heat exchangers in depth. Flow arrangements and pressure drop calculations were introduced in order to determine if the thermal design of the heat exchanger would be adequate in performance. Sources consulted Counter‐current and co‐current flow in heat exchanger. (n.d.). https://commons.wikimedia.org/wiki/File:Delta_T_1.png [Accessed 26 September]. Double pipe heat exchanger. (n.d.). https://commons.wikimedia.org/wiki/File:Double‐ Pipe_Heat_Exchanger.png [Accessed 26 September 2017]. Eagle, A & Ferguson, RM. 1930. On the coefficient of heat transfer from the internal surfaces of tube walls, Proceedings of the Royal Society of London A, 127:540. 42 Perry, RH & Green, DW. 2008. Perry's chemical engineers' handbook. New York: McGraw‐Hill. Plate heat exchanger. (n.d.). https://goo.gl/MwPPhs [Accessed 26 September 2017]. Principal components of a plate fin heat exchanger. (n.d.) https://en.wikipedia.org/wiki/File:Principal_Components_of_a_Plate_Fin_Heat_Exchanger.jpg [Accessed 26 September 2017]. Spiral heat exchanger schematic workaround. (n.d.). https://en.wikipedia.org/wiki/File:Spiral‐heat‐ exchanger‐schematic‐workaround.svg [Accessed 26 September]. Straight tube heat exchanger 1‐pass. (n.d.). https://commons.wikimedia.org/wiki/File:Straight‐ tube_heat_exchanger_1‐pass.PNG [Accessed 26 September 2017]. Towler, G & Sinnott, RK. 2013. Chemical engineering design: principles, practice and economics of plant and process design. 2nd edition. Oxford: Butterworth‐Heinemann. Tube arrangements. (n.d.). https://commons.wikimedia.org/wiki/File:Uk%C5%82ad_rurek_w_p%C5%82aszczowo‐ rurowym_wymienniku_ciep%C5%82a.png [Accessed 26 September 2017]. U‐tube heat exchanger. (n.d.). https://commons.wikimedia.org/wiki/File:U‐ tube_heat_exchanger.PNG [Accessed 26 September 2017]. 43 44 Learning unit 4: Separation tower design 4.1 Introduction The petroleum and chemical industries make extensive use of distillation processes to separate liquid mixtures into their individual components. The process of distillation relies on the differences in volatilities of the components to separate a mixture made of two or more liquids. The mixture is heated until one of the components turns to vapour. The vapour is then fed to a condenser where it is turned back to liquid by cooling down, and is known as the distillate. The component that remains in the original container is known as the residue. Many oil refineries use fractional distillation in order to purify crude oil so that it may become useful and produce a diversity of oil products suitable for many different applications. Distillation on an industrial scale is employed in South Africa by all the oil refineries (BP/Shell and Engen in Durban, Caltex in Cape Town and Natref in Sasolburg), industrial chemical manufacturers (Sasol, Safripol, Omnia) as well as some of the precious metal refineries (Impala, Rand Gold Refineries, etc.). Due to significant energy and capital requirements for distillation column installations, it is essential for chemical engineers to properly understand the underlying theory and practice for correct operation, maintainability and profitability. This learning unit will focus on developing concepts so that you understand the hydraulic design of a column. The hydraulic design of a distillation column encompasses the design of column internals. This would be classified as plates, distributors, packing supports, specifying suitable tray spacing, etc. In this unit we will first deal with some fundamental concepts that are important in distillation, and then describe the different types of trays and their functions in different applications. We will discuss the hydraulic design of a sieve tray in some depth, and lastly we look at the design of a packed column. You learnt about determining the stage and reflux requirements as well as sizing of the column in earlier modules. Should you wish to recap this content, please see chapter 17, sections 17.2 to 17.5 in the prescribed textbook. 4.2 Learning outcomes At the end of this learning unit, you should be able to define a tray tower select between a packed and tray column explain the physical layout and different components of a tray tower describe the different types of trays and their functions in different applications carry out an optimised hydraulic design of a sieve tray column explain the physical layout and the different components in a packed tower carry out a hydraulic design of a packed column with random packing 4.3 Tray towers: some general definitions Reading: To complete this unit, study chapter 17, section 17.13, in your prescribed textbook, Chemical engineering design. 1 A tray tower consists of a vertical column with the liquid phase flowing downward and the vapour phase flowing upward. The tower consists of trays or plates. The purpose of the tray is to allow contact between the liquid and the vapour. Each plate constitutes a single stage where liquid is retained on the tray, while vapour bubbles up through the holes in the tray. Tray columns are preferred in the following applications: The required liquid flows would be too low to maintain sufficient wetting of the packing. Generally, trayed columns can be designed with a greater level of confidence relative to packed columns, as it is easier to predict liquid distribution patterns for trays than for packing. The gas velocity is too low (or the diameter of the column is greater than 2 m). The solids are carried together with the gas, as the presence of a liquid layer on the surface of the trays allows for both the capturing of the solids as well as the continuous cleaning of the surfaces, which is not possible in packed columns. Furthermore, trayed columns are easier to clean. Highly exothermic chemical reactions occur during the absorption process (chemisorption), which would require intermediate cooling. Cooling coils can be installed in tray columns. It is easier to have side streams in a tray column. 4.3.1 Factors determining column performance: tray stability The main requirements of a tray are that it should provide good contact between liquid and vapour streams, so that the vapour and liquid leaving a tray are in equilibrium (or as close as possible to equilibrium conditions) operate satisfactorily (within stability and pressure drop limits) have sufficient area and space to handle the liquid and vapour flow rates The region where the tray operates at satisfactory conditions occurs over a limited range of liquid and vapour flow rates. Figure 17.33 in the prescribed textbook shows a diagram of the area for satisfactory tray operation. At low liquid rates, the liquid is pushed away from the holes in the tray by the vapour, resulting in a loss of efficiency due to poor liquid‐vapour contact. This is known as coning. Low vapour rates result in liquid seeping through the holes in the tray, which is known as weeping. Excessive weeping results in dumping – where none of the liquid reaches the downcomer. At high vapour rates the vapour bubbles carry liquid droplets to the plate above, resulting in a drop in efficiency. This is termed entrainment. Excessive entrainment results in instability in the column (drastic decrease in efficiency and high pressure drop) and this is termed flooding. At high liquid rates, the downcomer will reach its capacity and the liquid will accumulate on the tray above, resulting in downcomer backup flooding. The following clips show good visual simulations of flooding and entrainment in a distillation column: Flooding: https://www.youtube.com/watch?v=6Olr_IliW‐k Entrainment: https://www.youtube.com/watch?v=q7u3NkpeatY 2 Activity 4.1 1. Which condition sets the maximum and minimum vapour flow rate in the column? 2. Will a larger or smaller column diameter minimise entrainment? 3. Should we increase or decrease the spacing between the trays to avoid downcomer backup? 4.3.2 Tray types The most common class of trays are cross‐flow trays. A cross‐flow tray consists of a perforated tray deck across which the liquid flows and exits the tray via a weir and a downcomer, which is a vertical channel connecting one tray to another. The vapour flows up through the perforations in the tray. A typical cross‐flow layout is shown in figure 4.1 here or figure 17.23 in the prescribed textbook. Figure 4.1: Typical cross‐flow tray (Source: https://upload.wikimedia.org/wikipedia/commons/f/fb/Bubble_Cap_Trays.PNG) Other classes of trays include parallel flow trays, baffle trays and dual flow trays (which have no downcomers). These and other non‐cross‐flow trays are used for special purposes, for example when a low‐pressure drop is required or for handling fouling, corrosive and slurry services. Let’s look at the three types of cross‐flow trays commonly used in more detail. 4.3.2.1 Sieve trays Sieve trays are the simplest type of cross‐flow trays and consist of a perforated tray deck with holes of diameter from 1 mm to 25 mm. The vapour goes upward through the perforations in the tray and bubbles vigorously through the liquid on the plate. The weir ensures adequate liquid level on the plate for good vapour‐liquid contact. The liquid flows down in the downcomer to the tray below. The vapour rate should be adequate to prevent liquid from weeping through the perforations. The downcomer extends to below the liquid level of the tray below to make a liquid seal, preventing vapour from bypassing through the downcomers. A typical sieve tray is shown in your prescribed textbook, figure 17.24. The following video clips provide a good visual simulation of the sieve tray in the tower: 3 https://www.youtube.com/watch?v=OZIe__HB7Y0 https://www.youtube.com/watch?v=kBqr60kqo9U 4.3.2.2 Bubble cap trays Bubble cap trays are the oldest form of cross‐flow trays used in the distillation industry. They consist of a flat perforated plate with chimney‐like pipes (called risers) around the holes and caps in the form of inverted cups over the risers. This is illustrated in figure 17.25 in the prescribed textbook. Liquid and froth are trapped on the plate to a height of at least the chimney pipe height, giving this type of tray the unique feature of operating at very low liquid and vapour rates. View this video clip which explains the operation of a bubble cap tray: https://www.youtube.com/watch?v=6_3HxK9ruOM See a bubble cap tray undergoing inspection in the industry by following this link: https://www.youtube.com/watch?v=QzP6d_0QrZE 4.3.2.3 Valve trays Valve trays can be regarded as a cross between bubble cap and sieve trays. The construction is similar to that of bubble cap types but there are no risers and slots. They can also be described as a sieve tray with large diameter holes covered by adjustable flaps which lift as the vapour flow increase (floating valve trays). Floating valves can be rectangular or round. Fixed valve trays, however, are not adjustable. As the area for vapour flow varies with flow rate, valve plates can operate efficiently at lower flow rates than sieve plates. As the vapour rate falls, the openings are reduced or even closed. This limits weeping, which gives the valve tray its main advantage: a high turndown. Figures 17.26 and 17.27 in your prescribed textbook show a valve tray. View this video clip which explain the operation of a valve tray: https://www.youtube.com/watch?v=BdsM3bboeFM See pictures of various trays at the following manufacturer’s website: http://www.wermac.org/equipment/distillation_part2.html The liquid flow over the cross‐flow tray can be further classified according to the number of passes (figure 17.28 in the prescribed textbook): Single pass: This is the most commonly used arrangement. Reverse flow: The liquid flow is reversed using a central baffle on the plate. It is suitable for low liquid to vapour ratio. Multiple passes (two‐pass or four‐pass): The liquid is split in multiple streams. It is suitable for a high liquid to vapour ratio. Most trays are installed on support rings welded into columns. Smaller trays may be of the cartridge type or wedged between column flanges. In larger towers (> 2 m), trays will usually have support beams. Manways will allow people to travel from one tray to another. Manway sizes are usually 400 mm x 500 mm. The construction of plate absorption towers is similar to that of the plate distillation columns. Plate types differ depending on liquid and gas loading, the allowable pressure drop and extent of fouling anticipated – therefore, the 4 approach is very similar to distillation design. Historically, sieve trays and bubble cap trays were used in these applications. Presently, valve trays have replaced bubble cap trays in most applications as they are much simpler to manufacture and are therefore significantly cheaper. Sieve trays are still a reasonable choice in many duties, particularly as their typical disadvantages are less pronounced in absorption service. Selection of the correct tray design is usually based on some form of piloting or relies on vendor expertise. 4.3.3 Selection of plate type The main factors to consider when comparing the performance of bubble caps, sieve and valve plates are as follows (Towler & Sinnott 2013): 1. Capacity The capacity rating (the diameter of the column required for a given flow rate) of the three types is very similar; the ranking from highest to lowest is sieve, valve and bubble cap. 2. Efficiency The efficiency of the three types of plates is almost the same when operating over their design flow range. It is difficult to distinguish between the three based on efficiency. 3. Pressure drop Sieve plates usually give the lowest pressure drop. Valve trays have a lower pressure drop than bubble cap trays. 4. Cost Sieve trays are the most inexpensive. Bubble caps are usually the most expensive of the three trays. For mild steel trays, the ratio of costs of bubble cap:valve:sieve is 3:1.2:1. 5. Operating range Operating range refers to the range of vapour and liquid rates over which the plate will operate within stability limits. We normally refer to a turndown ratio, which is the ratio of the highest to lowest flow rates. Bubble cap trays provide the best turndown (they operate efficiently at low vapour rates). Valve plates have a better turndown than sieve trays. 4.3.4 Plate efficiency When considering multistage processes, it is normal to consider the theoretical equilibrium stage in order to simplify the mathematical analysis of the process. To compensate between the real contacting stages and the theoretical equilibrium stage, the concept of stage efficiency is introduced. Murphey (1925) proposed three principles of efficiency: 1. Murphey plate efficiency expressed as a ratio of actual separation to what would be achieved in an equilibrium stage: Where is the equilibrium composition of vapour leaving the plate. 2. Murphey point efficiency: vapour and liquid composition taken at a point on the plate. 3. Overall column efficiency: For an ideal situation, the overall column efficiency and the plate efficiency are related. The overall equation devised by Murphey is not practical as it does not give consistent slopes for the equilibrium and operating lines. The 5 O’Connell correlation gives better results, and is the preferred method used in estimating stage efficiency for distillation: 51 32.5 Where: = average molar liquid viscosity, mNs/m2 = average relative volatility of the light key For absorbers: 0.062 0.062 Where: = Henry’s law constant, Nm‐2/ mol fraction = total pressure, N/m2 = solvent viscosity, mNs/m2 = molecular weight of the solvent = solvent density, kg/m3 = equilibrium constant for the solute Study example 17.5 in the prescribed textbook. Activity 4.2 Compare sieve, valve, bubble cap and dual flow trays in a table with regard to the following features: • • • • • • • • • • • • • capacity efficiency turndown entrainment pressure drop cost maintenance fouling tendency effects of corrosion availability of design information main applications share of the market plate efficiency 4.3.5 General sieve tray design procedure The procedure for sieve tray design is outlined here and is recommended by Towler and Sinnott (2013). The procedure is intended for a preliminary design. The following areas are important for the tray design: 6 Total cross‐sectional area ( Downcomer area ( Net area ( ): Inside cross‐section area of tower. ): Downcomer cross‐sectional area (usually 12% of column area for a start). ): Cross‐sectional area less downcomer area (area available for vapour flow between trays). Active area ( ): Sometimes referred to as the bubbling area, this is the total cross‐sectional area less the total downcomer area, downcomer seal area and any other non‐perforated areas. This is usually approximated as: 2 . Hole area ( ): The total area of perforations (usually 10% of , for a first guess). 1. Calculate or estimate the vapour and liquid flow rates and the system physical properties of each of the following trays: • • • • top tray bottom tray the trays below and above any feed or draw‐off points any tray in the column on which the liquid or vapour loads peak Some property estimation methods are discussed in Chemical engineering design, section 17.3. In an examination, you will be given the required properties. To calculate the required vapour and liquid rates, refer to your separation principles (distillation and absorption) module. In practice, these will normally be determined with the use of a simulation package such as Aspen Plus. A turndown ratio is normally required as well in order to determine the maximum and minimum flow rates. In the exam all of this information will be provided, but you will not be so fortunate in real life! The actual number of required trays should also be estimated prior to the next steps to ensure that the pressure drop requirements over the entire tower are met. The actual number of trays (as opposed to the theoretical number of trays) should be rechecked after the tray design, because the actual tray design will influence the tray efficiency which, in turn, affects the actual number of trays. 2. Select a trial tray spacing The distance between two trays is known as the tray spacing. The tray spacing will determine the overall height of the column. The plate spacing varies from 0.15 m to 1 m. The spacing selected is a function of column diameter and the type of service (fouling, corrosive, foaming). Columns with a small diameter would require a smaller spacing, whereas large diameter columns would require bigger plate spacing to accommodate the support structure of the column. A reasonable initial estimate for the tray spacing is between 450 mm and 600 mm. Towler and Sinnott (2013) suggest 500 mm (0.5 m). 3. Estimate the column diameter The column diameter can be estimated based on the flooding velocity, i.e. the upper limit to the vapour velocity. The vapour velocity is assumed to be 80‐85% of the entrainment flooding velocity. 7 The flooding velocity can be calculated by using the method of Fair as described by Towler and Sinnott (2013). Where: uf = flooding vapour velocity, m/s, based on the net column area An K1 = a constant obtained from figure 17.34 in the prescribed textbook To read off K1 from figure 17.34, you would need to know the liquid‐vapour flow factor: Where: Lw = liquid mass flow rate, kg/s Vw = vapour mass flow rate, kg/s The following restrictions apply to the use of the flooding velocity chart (figure 17.34): 1. Hole size less than 6.5 mm; entrainment may be greater with larger hole sizes 2. Weir height less than 15% of the plate spacing 3. Non‐foaming systems 4. Hole:active area ratio greater than 0.10; for other ratios apply the following corrections: Hole:active area Multiply K1 by 0.1 1 0.08 0.9 0.06 0.8 5. Liquid surface tension 0.02 N/m, for other surface tensions, σ, multiply the value of K1 by [σ/0.02]0.2. Based on the velocity (80‐85% of the flooding velocity) and the vapour flow rate on the tray, an estimate of the net area can be made. Before you can obtain the total tower cross‐sectional area, you also need to calculate the downcomer area ( ). Towler and Sinnott (2013) use 12% of the column area as a first estimate for the downcomer area: 12 100 12 88 8 A more sophisticated approach is proposed by Kister (1992) in which the downcomer area is based on a recommended liquid velocity: Recommended velocities are listed in table 4.1. Table 4.1: Maximum downcomer velocities Foaming tendency Example Low Clear liquid velocity in downcomer (m/s) 450 mm 550 mm 650 mm Low pressure light hydrocarbons (<6 bar) water‐air systems 0.12 – 0.15 0.15 – 0.18 0.18 – 0.21 Medium Oil systems, crude oil distillation, absorbers, mid – pressure hydrocarbons (6 to 20 bar) 0.09 – 0.12 0.12 – 0.15 0.15 – 0.18 High Amine, glycerine, glycols, high pressure light hydrocarbons (>0 bar) 0.06 – 0.075 0.06 ‐0.075 0.06 – 0.09 Kister (1992) recommends that 75% of these velocities be used for conservative design. The required tower cross‐sectional area can now be calculated ( ) and the diameter can be calculated based on this area. If the calculated diameters for the different trays differ by more than 20%, it may be more economical to build the column in sections with different diameters. 4. The next step is to select the liquid flow pattern, i.e. single pass, double pass, reverse flow, etc. Figure 17.35 in the prescribed textbook gives a guide for the selection of this (based on the column diameter and liquid flow rate). Kister (1992) recommends that a liquid flow pattern be selected to ensure that the liquid flow rate over the downcomer weir is in the range of 63 to 120 m3/hr per m of weir. 5. Enough information is now available to do a preliminary tray layout (sections 17.13.8 to 17.13.11 in Chemical engineering design). At this stage you will have values (although they may be rough estimates with plenty of optimisation needed) for the following: • • • • • • • • • • column diameter column area ( ) downcomer area ( ) net area ( ) active area ( 2 ) hole area (10 % of , for first estimate) weir length ‐ normally between 0.6 and 0.85 of the column diameter (As a first guess use 0.77 which is equivalent to a downcomer area of 12%. For segmental downcomers the length of the weir fixes the area of the downcomer.) weir height ‐ determines the volume of liquid on the plate; for columns operating above atmospheric pressure, weir height is 40 mm to 90 mm, whereas for a vacuum operation 6 to 12 mm is within range Hole diameter ‐ 5 mm for clean services and 12 mm for fouling services Plate thickness ‐ according to Chemical engineering design, 3 mm for alloy steel and 5 mm for carbon steel 9 You still have to check if the tray will be operating satisfactorily with regard to the tray stability diagram, the allowable pressure drop, the required turndown ratio and the required downcomer residence time. You also have to optimise the design in terms of tray spacing, number of holes, hole pitch, hole size and calming zones. The optimised tray has to be checked for the above requirements. 6. Check the weep point and thus the actual turndown ratio. The weep point occurs when leakage through the plate holes becomes excessive. To avoid weeping, the hole area must be chosen so that vapour rate velocity is above the weep point. The minimum design vapour velocity is given by: 0.90 25.4 Where: uh = minimum vapour velocity through the holes (based on the hole area), m/s dh = hole diameter, mm K2 = a constant, dependent on the depth of clear liquid on the plate, obtained from the weep point correlation in figure 17.37 in the prescribed textbook Actual minimum vapour velocity= Minimum vapour rate Ah Weir‐liquid crest 750 Where: lw = weir length how = weir crest, mm liquid Lw = liquid flow rate, kg/s The weep rate can be reduced by reducing the hole area or decreasing the individual hole diameters. 7. Check the total tray pressure drop (Chemical engineering design, section 17.13.14). As before, hole diameters and hole area will affect the pressure drop. To calculate the total pressure drop, the vapour flow through the dry plate (hd) needs to be calculated as well as the head of clear liquid ( on the plate. Other minor pressure losses are accounted for by the residual head loss (hr). Therefore: Total plate pressure drop And 10 51 12.5 10 Where C0 is the orifice coefficient obtained from figure 17.42 in the prescribed textbook. 8. Check the downcomer backup and the downcomer residence time (Chemical engineering design, section 17.13.15). Where: = residence time, s = clear liquid backup, m = liquid flow rate in downcomer, kg/s A time of at least 3s is recommended. Kister gives the following additional guidelines for required downcomer residence time: Low foaming tendency: 3 seconds’ residence time Medium foaming tendency: 4 seconds’ residence time High foaming tendency: 5 seconds’ residence time Very high foaming tendency: 7 seconds’ residence time A downcomer backup of less than half the tray spacing plus the weir height is recommended. The tray spacing may be reduced at this stage if possible while still satisfying this requirement. The downcomer area may also be adjusted. 9. At this stage some parameters might have changed that will affect entrainment flooding and they therefore have to be checked again. The column diameter may be adjusted (increased or decreased). 10. Steps 5 to 10 may need to be repeated until all requirements are met and the design is optimised in terms of column diameter and plate spacing. Figure 4.2 summarises the sieve tray design procedure. 11 Calculate vapour and liquid flow rates from mass balance Calculate plate pressure drop Check downcomer backup, if high carry out trail plate layout calculations or choose new plate spacing Collect Physical properties (or estimate using interpolation) Check weeping rate Plate layout details: calming zones, unperforated areas etc. Select a trial plate spacing Trial plate layout: downcomer area, active area, hole area and size, weir height Adjust column diameter bases on % flooding Accept design if all criteria met Estimate column diameter Determine liquid flow arrangement Optimise design Finalise design and sketch layout Figure 4.2: Algorithm for sieve plate design Example 4.1 Study example 17.6 in the prescribed textbook. The procedure for tray tower design is presented in example 17.6 in Chemical engineering design. The aim of this procedure is to point out that the example presented in the prescribed textbook (example 17.6) should be used with caution for real‐life designs and that there are more factors that need to be considered. For examination purposes, it would be acceptable to use the procedure and equations as per the prescribed textbook. As example 17.6 is already clearly presented in the textbook, it will not be repeated in this guide. We recommend that you work slowly through the textbook example and also note the following comments, which refer to the textbook example: 1. All required information is given or calculated in the example. 2. As the system has a low foaming tendency, the downcomer area can be calculated as the volumetric flow rate divided by 75% of the recommended liquid velocity (0.15 m/s) as stated in the procedure. This gives an area of 0.03 m2, which is 6.5% of the column area. Thus, 12% as per the textbook may be too conservative. 3. Total area = 0.49 m2. Thus column diameter = 0.79 m. The bottom tray and top tray differ by more than 20%, so it may be considered as an option to make the bottom and top sections in different diameters. 4. A single‐pass plate is selected. For a weir width of 0.6 m, this translates into a flow of 21.2 m3/h per m of weir, which is well within the recommended range. 5. Plate layout as per prescribed textbook. 6. Weeping checked for 70% turndown and found to be acceptable. 7. Pressure drop acceptable. 8. Residence time in downcomer is acceptable, as the system is not foaming. 9. lt may not have a major effect on the column diameter to reduce the downcomer area. It might have quite an effect on pricing to reduce the area of the top section and we recommend that you investigate further. 12 Activity 4.3 1. Design a sieve tray for the following service: Vapour rate 14 860 kg/h Liquid rate 12 100 kg/h Vapour density 2.01 kg/m3 Liquid density 807 kg/m3 Surface tension 0.0263 N/m Assume a 0.5 m tray spacing and downcomer area as 12% of the column area. Design for 80 ‐ 85% flooding. The pressure drop for the tray should not exceed 150 mm of water. Your design should include a provisional plate design, liquid flow arrangement, check for weeping and entrainment, tray pressure drop, downcomer backup, residence time and final plate layout details (with plate specification and layout diagram). 2. An existing single‐pass sieve tray (dimensions listed here) is to be evaluated for a given service. The design requires a vapour velocity which is 75 ‐ 80% of the flooding value and a turndown ratio of 70%. Column diameter (m) Downcomer area (m2) Hole area Hole diameter (mm) Weir height (mm) Weir length (m) Tray spacing (m) Tray thickness (mm) Conditions and properties at the tray are as follows: Vapour rate 1.5 12% of total area 10% of active area 5 50 1.155 0.45 5 15 600 kg/h Liquid rate 14 750 kg/h Vapour density 1.64 kg/m3 Liquid density 1 079 kg/m3 Liquid viscosity 0.7 cP Surface tension 0.029 N/m Based on the given diameter (please do not calculate a diameter; use the diameter specified above), determine whether the specified liquid arrangement is suitable. Determine the flooding velocity and the actual velocity based on the net area. Determine whether entrainment and weeping will be an issue. Estimate the tray pressure drop and state whether it is acceptable or not. Determine whether the tray spacing is acceptable by calculating the downcomer back‐up. Determine whether the residence time is acceptable. 13 4.4 Packed towers: definitions, packing types and internals Reading: To complete this unit, study chapter 17, section 17.14, in your prescribed textbook, Chemical engineering design. 4.4.1 What does a packed tower look like? The different components of a packed vapour‐liquid separation tower are shown in figure 4.3. Figure 4.3: Packed tower (Source: https://commons.wikimedia.org/wiki/File:Packed_bed_column.svg) Packing can generally be divided into three classes: • • • random packing structured packing grids The use of grids is usually limited to particular applications; therefore we will discuss only the first two classes in this guide. Vapour‐liquid contact is achieved in the packed part, and the other parts only have the function to keep the packing in place and to make sure it performs efficiently. For the packing to perform efficiently, the liquid has to be distributed evenly over the packing surface. The vapour has to be spread evenly over the entire vapour flow area. The larger the vapour‐liquid contact surface, the higher the packing efficiency. 14 Even liquid distribution is achieved through liquid distributors and liquid redistributors. A support grid at the bottom supports the packing. Bed limiters or hold‐down grids at the top of packed sections ensure that the packing is kept in place. Sometimes even vapour distribution is achieved by use of a vapour distributor. The collective term for all these items ensuring that the packing performs efficiently is internals. Watch the following clip: https://www.youtube.com/watch?v=AzK7K601cAE In the next section you will learn about random and structured packing. Because the choice of the correct internals for a specific application is imperative for satisfactory operation, we will discuss internals in more detail in section 4.4.3. It is generally recommended that the supplier of the packing also supply the internals. According to Towler and Sinnott (2013), the principal requirements for packing are the following: • • • • Provide a large interfacial area between the gas and liquid. Have an open structure and thus provide a low resistance to gas flow and a low pressure drop. Promote uniform liquid distribution on the packing surface. Promote uniform vapour gas flow across the column cross‐section. 4.4.2 Random and structured packing 4.4.2.1 Random packing Today random packing is generally available in most metals (carbon steel, stainless steel, copper and exotic alloys), plastic (polypropylene, polyethylene, PVDF), ceramics and carbon. Random packing consists of many individual elements, ranging from hollow cylinders to specially designed elements, usually of vendor‐specific design. Some examples are shown in figure 4.4. (a) (b) (c) Figure 4.4: Random packing sizes Sources: (a) Raschig rings (n.d.) https://commons.wikimedia.org/wiki/File:RaschigRings005.JPG (b) Anelli Raschig (n.d.) https://commons.wikimedia.org/wiki/File:Anelli_Raschig.jpg (c) Bialecki rings (n.d.) https://commons.wikimedia.org/wiki/File:Bialecki_rings.jpg Figure 17.46 in your prescribed textbook also provides a range of different types of packing available industrially. The following link provides a good animation of random packing in a tower: https://www.youtube.com/watch?v=yoWNau1Fqak 15 4.4.2.2 Structured packing Structured packing has been available since the early 1940s. There have been different stages of development. An example of structured packing is shown in figure 4.5. Properties of structured packing are as follows: • large specific packing surface complete and equal wetting of the packing surface combined with a continuous renewal of the liquid film good distribution of vapour flow Figure 4.5: Source: https://commons.wikimedia.org/wiki/File:Riempimento_strutturato.jpg To view a structured packing demonstration, follow the link: https://www.youtube.com/watch?v=p5S6Ri5aemA Kister (1992) gives a comprehensive discussion of the different types of random and structured packing as well as a comparison, which is summarised here. Comparison between random and structured packing • • • • • • Structured packing shows superior performance at low liquid rates. Structured packing offers a far greater specific surface area. At higher liquid rates the effect of the above advantages for structured packing fades rapidly. Structured packing has a much lower pressure per theoretical stage than random packing, which is a major advantage in vacuum applications. In high pressure applications structured packing tends to perform poorly for reasons which, at this stage, are not very well understood. Random packing is generally cheaper than structured packing per volume, but a smaller volume of structured packing may be required. 4.4.3 Internals 4.4.3.1 Liquid distributors and redistributors The performance of a packed bed can deteriorate by a factor of 3 because of liquid maldistribution, and therefore we cannot emphasise the correct design and choice of liquid distributors enough. Figure 4.6 shows the effect of liquid maldistribution. 16 Figure 4.6: Effects of liquid maldistribution To achieve this, liquid distributors are used directly above the packed bed, below the point of liquid entry into the absorption column. Designs usually vary based on throughput, with the following designs being most common: Orifice type: These are very similar to perforated/sieve trays, providing relatively good wetting of the packed bed. They must not be used if there is a possibility of plugging the holes (such as if there are solids present or forming during contact with gas). They may allow for easy entrainment (carryover) of liquid, so are not suitable for high gas rates. Notched chimney type: These are suitable for medium to high throughputs and are not prone to blockages. Notch‐through distributors: These are suitable for larger towers and higher gas rates because of their large free area (which makes entrainment more difficult). Perforated ring type: These are suitable for high gas flow rates and relatively small liquid rates. See figures 17.59 to 17.62 in your prescribed textbook for drawings of the different types of liquid distributors. You could also view some of the images on the following manufacturer’s website: https://www.amacs.com/fractionation‐tower‐internals/distributors/ Currently, pressure devices (spray nozzles, spider pipes and perforated rings) are rarely used because dirty liquids easily block their small orifices. In addition, the fine particle spray that they produce is easily entrained in the gas stream. Liquid redistributors are required for the introduction of an intermediate feed in a packed column. They are also required to redistribute larger streams that gradually form as the liquid trickles downwards over the packed bed. Liquid distributors also remove liquid from the wall and redirect it to the centre of the bed. Kister (1992) recommends a liquid redistributor for at least every 6 m of packing. Read more about the importance of liquid distributors by following the link: http://www.cewindia.com/sameer_mehta_features.html 4.4.3.2 Flashing feed distributors According to Kister (1992), introducing a vapour‐containing feed in a distributor for liquid feeds only can severely lower column efficiency. In a flashing feed distributor, the vapour and liquid have to be separated prior to liquid distribution. Different types of flashing feed distributors are explained in more detail in the manufacturer’s brochure: http://www.koch‐glitsch.com/Document%20Library/Metal_Packing_Feed_Devices.pdf 4.4.3.3 Vapour distributors 17 Vapour distributors are located at or above a vapour feed, between a trayed and a packed section or above the transition section where there is a change in tower diameter, according to Kister (1992). The following devices may be used: • • • a sparger pipe a vapour distributor (most of the time a chimney tray) a vapour‐distributing support 4.4.3.4 Packing supports Kister (1992) lists the following as functions of packing support grids: • Provide physical support of the packed bed. • Incorporate sufficient open area in order to permit unrestricted flow of vapour and liquid. • Avoid downward migration of pieces of packing. It is recommended that the open area of packing supports be at least 70 to 100% of the column cross‐sectional area to avoid vapour or liquid bottlenecking. These are the common types of packing supports: Gas injection supports: In metallic and plastic random packing applications this type of support is the least likely to become a capacity bottleneck. Open areas of approximately 100% are common. Grid supports: Grid supports are generally less expensive than gas injection supports. Sometimes packing may block some of the open area, which will bottleneck column capacity. It may provide insufficient open area with small packing sizes. Open areas of as high as 70% of the column cross‐sectional area are used for ceramic packing applications. 95% to 97% is used for metallic applications. Grid supports are normally used for structured packing. See the gas injection type support as well as a grid support in the manufacturer’s brochure: http://www.koch‐ glitsch.com/Document%20Library/Metal_Packing_Support_Plates.pdf 4.4.3.5 Hold‐down plates and bed limiters Hold‐down plates are used with ceramic or carbon random packing to prevent fluidisation and restrict packing movement, which may break packing particles. For metal or plastic random packing, bed limiters are used. Unlike hold‐down plates, bed limiters do not rest on the packing, but are fastened to the column with a support ring or bolting clips. For both of these an open area of at least 70% of the column cross‐sectional area is recommended. 4.4.4 General packed tower design procedure Most modern column packing is proprietary. Column sizing is an interactive process between the end user and the supplier. Here is a preliminary procedure for packed tower design. 1. Calculate or estimate the vapour and liquid flow rates and the system physical property profiles through the column. We recommend that you do your design for each of the following regions in the column: • • • • top of column bottom of column below and above any feed or draw‐off points at any point in the column where the liquid or vapour flow rate peaks 18 The theoretical number of stages will also be required to calculate the required height of the packed bed. Factors that may affect the design, such as solids contents, fouling and corrosion, will have an effect on the choice of packing and should be taken into account. 2. The Height Equivalent of a Theoretical Plate (HETP) concept is used to calculate the required packed height. At this stage a specific type of random packing or structured packing should also be selected. Packing suppliers should be able to give guidelines. The packed height is calculated by multiplying the HETP by the required number of theoretical stages. Some designers prefer to use the NTU (number of transfer units) concept to calculate the required height. This concept is discussed in more detail in the prescribed textbook, section 17.14.2, and you should have covered it in earlier modules on separation principles. The basic equations are summarised here: To simplify calculations, the separation principles in packed beds are treated as a staged process with the height of an equilibrium stage used to convert the number of ideal stages into the height of packing required. This is calculated as follows: 1 Where: = height of the overall gas‐phase transfer unit = molar gas flow rate per unit cross‐sectional area = molar liquid flow rate per unit cross‐sectional area 3. The next step is to calculate the diameter of the column. The column should be designed to operate at the most economical pressured drop in order to ensure a good liquid and gas distribution in the column. This depends on the packing size and the packing factor. The packing size is influenced by the column diameter and using an excessively large packing size would result in poor liquid distribution. Recommended ranges from Towler and Sinnott (2013) are as follows: Column diameter Recommended packing size <0.3 m < 25 mm 0.3 to 0.9 m 25 to 38 mm >0.9 m 50 to 75 mm Recommended design values given as mm water per mm packing (mmWG/m) are as follows: Absorbers and strippers 15 to 50 mmWG/m Distillation, atmospheric and mod pressure 40 t0 80 mmWG/m Pressure drop across the packed section is an important consideration in absorption system design due to associated energy costs for compression of the feed gas. Packing material manufacturers typically supply pressure drop charts, such as the one shown in figure 17.54 in the prescribed textbook, to assist with the determination of pressure drop in the figure is defined as follows: in the tower. The term 19 13.1 . ∗ Where: Vw* = gas mass flow rate per unit column cross‐sectional area, kg/m2s Fp = packing factor, characteristic of the size and type of packing, m−1 μL = liquid viscosity, Ns/m2 ρL, ρv = liquid and vapour densities, kg/m3 In terms of flooding, columns are normally sized for 75% of flooding. At flooding the vapour rate is so high that it carries the liquid upwards in the column, resulting in a dramatic increase in pressure drop and column instability. factor: The percentage flooding could be calculated from the K4 at design pressure drop Percentage flooding= K4 at flooding 1 2 This criterion can be superseded by either of the following criteria: • • There may be a limitation on the total pressure drop of the packed bed. For example, the system may be operated under vacuum and a pressure drop higher than the specified pressure drop may lead to higher temperatures and product degradation in the column. There may be a limitation on the pressure drop of the packed bed per metre of packing in terms of the specific system. For example, if the process fluids are known to foam, the recommendation may be to limit the pressure drop to a certain value in order not to lose packing efficiency due to foaming. As with tray towers, if the calculated tower diameter differs by more than 20% for different sections, it may be economically feasible to design the column in sections with different diameters. 4. The minimum wetting rate is the lower stability limit for packed tower operation. If the liquid flow is below the minimum wetting rate, the falling liquid film breaks up, which causes de‐wetting of the packing. This reduces the vapour‐liquid contact area to such an extent that a dramatic reduction in efficiency is experienced. The normal operating liquid flow should be well above the minimum wetting rate. The minimum wetting rate is defined as: The range for minimum wetting rates for random and structured packing are as follows: Random packing 0.35 10 1.4 Structured packing 0.07 10 0.14 10 10 / / 5. Kister (1992) recommends the following as a minimum vapour flow guideline: The vapour flow should be enough to induce a minimum pressure drop of 8.3 mm H2O per m of packed bed. Although there are many columns in practice that operate below this pressure drop, vapour maldistribution (resulting in reduced mass transfer) may occur below this pressure drop. 20 6. The next step is to select and design the required column internals. As mentioned earlier, Kister (distillation operation) gives an excellent description of all the dos and don'ts for internal selection. It is very important that the packing supplier give their input and preferably supply the internals. Remember that use of a liquid redistributor is highly recommended for every 6 m of packed bed height. For exam purposes internal design will not be required, but internal selection will be required with specific reasons for the selection of the specific internals. 7. It may be necessary to go through the design process several times with a different type of random packing to optimise the packed tower economically. Activity 4.4 Compare trays, structured packing and random packing with regard to the following: • • • • • • • • • • • • vacuum systems cost revamps low pressure drop applications small‐diameter columns corrosive systems foaming systems solids large diameters weight performance prediction certainty turndown Example 4.2 Do a hydraulic design of a stripper column to strip H2S from an effluent stream by means of air to a composition of less than 10 ppm. The following information applies: Liquid rate: 9.2 m3/h Liquid density: 1 040 kg/m3 Liquid viscosity: 1 cp Surface tension: 0.07 N/m Vapour rate: 2 060 m3/h Vapour density: 1.1 kg/m3 8. Theoretical stages are required as per a simulation study. It is requested that the column be designed for only 65% of flooding capacity, because of a possible later expansion. Solution 1. All required information for the design is available. 2. For 8 theoretical stages and using 50 mm Intalox metal packing, with an expected HETP of 0.5 m, this translates into 4 m of packing. Choose a packed bed height of 5 m as a safety margin. 21 3. The printout of the software output from the website http://packed‐column‐calculator.soft112.com/ is presented in figure 4.7. The total expected pressure drop would be 31.5 mmWG/m =3.2 mbar/m x 5 m = 16 mbar or 1.6 kPa. The diameter of the column is 0.73 m. 4. The fan for sucking the air over the packing should be sized for this plus an additional 1.5 kPa for pressure drop over internals and ducting. The fan may be installed with a damper to throttle the vapour rate. 5. The liquid rate is 2.66 kg/s which is well above the minimum liquid rate of 0.38 kg/s. See figure 4.8 for detailed calculations. 6. The vapour rate is above the minimum required vapour rate. 7. The bed is only 5 m, thus no redistributor is required. A gravity‐type distributor should be used at the top to avoid entrainment (as with pressure distributors). A hold‐down plate is recommended on top of the packing. The packing is to rest on a gas injection support at the bottom to ensure that there is no vapour maldistribution as there are no feeds into the middle of the packing. Figure 4.7: Output from software 22 Figure 4.8: Detailed calculations Study example 17.9 in the prescribed textbook. Activity 4.5 1. You have been assigned to assess a packed tower with the following hydraulic data: Vapour rate 2.20 kg/s Liquid rate 1.80 kg/s Vapour density 5.20 kg/m3 Liquid density 720.0 kg/m3 Liquid viscosity 0.631 cP The diameter of the packed column is 0.85 m and the packing used is 1 inch metal pall rings. a) Calculate the percentage flooding. b) If the maximum allowed pressure drop for the column was specified at 600 Pa/m, determine whether the specified packing will achieve this. 23 2. A packed distillation column for the separation of methanol and water is to handle a liquid load of 10 000 kg/h and a vapour load of 21 000 kg/h. = 1.104 kg/m3 and = 0.35 cP. The fluid properties are: = 770 kg/m3; Design a packed column for 70% flooding by specifying column diameter packed height, given that the separation requires 8 theoretical stages liquid hold‐up pressure drop 4.5 Summary Having completed this learning unit, you should be able to determine whether trays or packing should be used in the tower design. The learning unit also provided background information that would enable you to explain the physical layout and different components in a packed tower. We discussed the hydraulic design of a sieve tray column in depth and that of a packed tower using random packing. We introduced additional factors such as tray tower selection, plate efficiency and general considerations for successful tower operation. Sources consulted Anelli Raschig. (n.d.). https://commons.wikimedia.org/wiki/File:Anelli_Raschig.jpg [Accessed 27 September 2017]. Bialecki rings. (n.d.). https://commons.wikimedia.org/wiki/File:Bialecki_rings.jpg [Accessed 27 September 2017]. Bubble cap trays. https://upload.wikimedia.org/wikipedia/commons/f/fb/Bubble_Cap_Trays.PNG [Accessed 27 September 2017]. Kister, HZ. 1992. Distillation design. New York: McGraw‐Hill. Murphey, EV. 1925. Rectifying column calculations. Journal of Industrial Engineering Chemistry, 17:747. Packed bed column. (n.d.). https://commons.wikimedia.org/wiki/File:Packed_bed_column.svg [Accessed 27 September 2017]. Packed column calculator. (n.d.). http://packed‐column‐calculator.soft112.com [Accessed 27 September 2017]. Raschig rings. (n.d.). https://commons.wikimedia.org/wiki/File:RaschigRings005.JPG [Accessed 27 September 2017]. Structured packing. (n.d.). https://commons.wikimedia.org/wiki/File:Riempimento_strutturato.jpg [Accessed 27 September 2017]. Towler, G & Sinnot, RK. 2013. Chemical engineering design: principles, practice and economics of plant and process design. 2nd edition. Oxford: Butterworth‐Heinemann. 24 Learning unit 5: Equipment design – transport of fluids 5.1 Introduction Almost every chemical process will require systems and equipment to transport fluids within the process. It is therefore important to understand the different types of equipment that may be used to achieve the movement of liquids and gases in the process. The design engineer would also need to provide the specifications for this equipment. This learning unit provides the theory required for the design of equipment used for transporting fluids such as pumps compressors expanders valves We will not deal with equipment used for transporting solids. For interest you could read chapter 18 in the prescribed textbook. Pumps are used in a wide range of applications in the engineering industry to transfer liquids from one unit to another. Compressors are used to increase velocity and pressure of gases. Expanders are used often in place of valves to recover energy when the pressure of a gas needs to be decreased. 5.2 Learning outcomes At the end of this learning unit, you should be able to select the appropriate type of pump and specify the necessary design data for the pump, such as flow rates, power requirements and efficiency differentiate between compressors and expanders differentiate between centrifugal and positive displacement pumps compute the work done by compression and expansion calculate and determine the appropriate valve size 5.3 Design of pumps Reading: Revise section 20.4 (pressure drop in pipelines) in the prescribed textbook before starting this section. You should have covered this material in previous modules. To complete this learning unit, study section 20.7 in your prescribed textbook. 5.3.1 Types of pumps Pumps are required to move liquids and gases as well as slurries from one point in the process to another. The movement of the fluid requires energy which is supplied either by electricity or steam. There are various types of pumps available commercially and understanding which pump is correct 1 for the process application is important in order to reduce costs and pump maintenance. We discuss two basic types of pumps in further detail. 5.3.1.1 Centrifugal pumps These are the most commonly used pumps in the chemical industry. A centrifugal pump is a type of pump with one or more impellers. Liquid enters in the middle of the impeller, which rotates, causing the liquid to be displaced to the outer edge of the impeller (centrifugal force). This causes the velocity of displaced liquid to increase. At the discharge of the pump, the kinetic energy (due to increased velocity) is converted into pressure energy. Therefore, the fluid enters the pump at a low pressure and exits with a high discharge pressure. The advantage of a centrifugal pump is that many different types of liquids can be pumped. It also handles lower viscosity liquids well and is suitable for high flow rates. Figure 5.1 shows a centrifugal pump. The following video clip also provides a good animation of how a centrifugal pump operates: https://www.youtube.com/watch?v=BaEHVpKc‐1Q Figure 5.1: Cutaway of a centrifugal pump (Source: https://commons.wikimedia.org/wiki/File:Centrifugal_Pump_en.svg ) 5.3.1.2 Positive displacement pumps A positive displacement pump relies on rotating or reciprocating parts to push the liquid into an enclosed volume until enough pressure is built up to move the liquid into the discharge pipe. A bicycle pump is the most basic form of a positive displacement pump which functions via a hand‐ operated piston. During the up‐stroke, the piston draws air through a one‐way valve into the pump from the outside. During the down‐stroke, the piston displaces the air from the pump into the bicycle tyre. A built‐in pressure gauge is used to determine the tyre pressure. Theoretically, a 2 positive displacement pump can produce the same flow rate at a given speed regardless of the discharge pressure. These types of pumps are used with variable speed drivers where power efficiency is important. Figure 5.2 shows a positive displacement pump. Figure 5.2: Positive displacement pump (Source: https://en.wikipedia.org/wiki/Pump#/media/File:Gear_pump.png ) Watch the following clip to gain a better understanding of the functioning of different types of positive displacement pumps: https://www.youtube.com/watch?v=ySbtksESFUc When deciding which type of pump to choose, it is important to consider the flow rate (or capacity) and head required. Process conditions of the fluid, such as viscosity, corrosive properties as well as the presence of solid particles in the fluid, need to be taken into account. Table 5.1 summarises the principal types of pumps used and their operating pressures and capacity ranges (Towler & Sinnott 2013). Table 5.1: Normal operating range of pumps Type Centrifugal Capacity range m3/hr Typical head (m of water) 0.25 ‐ 103 10 ‐ 50 300 (multistage) Reciprocating 0.5 ‐ 500 50 ‐ 200 Diaphragm 0.05 – 50 5 ‐ 60 Gear and similar 0.05 ‐ 500 60 ‐ 200 Sliding vane or similar 0.25 ‐ 500 7‐70 Rotary Rotary 3 The following websites also provide a guide to pump selection for a wide range of positive displacement and centrifugal pumps: http://www.pumpscout.com/articles‐scout‐guide/pump‐types‐guide‐aid100.html or http://www.globalspec.com/learnmore/flow_transfer_control/pumps/pumps_all_types Charts available in your prescribed textbook, Chemical engineering design ‐ figures 20.3 and 20.4 ‐ are useful in determining the type of pump required for a particular head and flow rate for a centrifugal pump and positive displacement pump, respectively. You can also see a pump selection guide on the following website: http://www.oilngasprocess.com/oil‐handling‐ surfacefacilities/pumps/basic‐pumps‐selection‐criteria.html Table 5.2 summarises the advantages and disadvantages of the different types of pumps. Table 5.2: Centrifugal vs positive displacement pumps Centrifugal pumps Positive displacement pumps Produces flow by creating pressure Produces pressure by creating flow Handles different liquids High power efficiency Suitable for high flow rates Good suction lift obtained by vacuum on the inlet side Handles low viscosity fluids well Flow rate increases with increasing viscosity Cavitation occurs easily if not run at the operating point on the curve Efficiency not affected by pressure, cavitation less likely to occur if run off the curve As centrifugal pumps are the most widely used type of pump in industry, we will discuss the design of this type of pump in further detail. 5.3.2 Centrifugal pump design A centrifugal pump converts rotational energy from the motor into energy in a moving fluid. Fluid enters the pump through the eye of the casing where it is whirled by the impeller blades outwards until it is discharged through the diffuser part of the casing. The impeller is connected at the shaft to a power source, causing the impeller to rotate at a high speed. The high speed of the impeller provides the fluid with a high kinetic energy which is converted to a high pressure as deceleration of the fluid at the discharge occurs. Figure 5.3 shows a schematic of a centrifugal pump. 4 Shaft Discharge Fluid Flow Impeller Casing Figure 5.3: Schematic of a centrifugal pump (Source: https://commons.wikimedia.org/wiki/File:Centrifugal_pump_volute_Richards_1894.png) The centrifugal pump has been designed to accommodate different sizes and types of impellers and is characterised by its speed. Pump‐specific speed ( ) is a method of characterising pumping conditions and it may be used to determine the most appropriate pump design for a given application. Often pump characteristics, such as suction lift, net positive suction head and pump efficiency, will be correlated against the pump‐specific speed. Pump manufacturers define the impeller speed of a centrifugal pump by the equation: / / Where: N Q flow, US h head, ft revolutions per minute rpm gal min The pump‐specific speed is not dimensionless; however, its dimensions are important for converting between different unit sets (e.g. converting and comparing pump‐specific speeds calculated on a metric basis and US customary basis). 5 Depending on the type of impeller, the speed of a centrifugal pump would lie between 400 and 20 000. Pump impellers are generally classified as follows: centrifugal/radial – speeds 400 – 4 000 mixed flow – speeds 4 000 – 9 000 axial – speeds ‐ > 9 000 5.3.3 Power requirements for pumping liquids Liquids are transported from one vessel to another via pipelines. Energy is required to move the fluid from one point to another. The energy required depends on the following: friction losses in pipelines miscellaneous losses in the pipe fittings including bends, valves and instruments losses accounting from process equipment such as heat exchangers and packed beds losses from elevation differences in pipelines pressure differences between vessels between pipelines The total work required is calculated from a mechanical energy balance for a liquid: ∆ ∆ ∆ 0 Where: W = work done by the fluid, J/kg Δz = difference in elevation height (z1 – z2), m ΔP = difference in pressure (P1 – P2), N/m2 ΔPf = change in pressure due to friction as well as miscellaneous losses such as bends etc. ρ = density of the liquid, kg/m3 g = acceleration due to gravity, m/s2 If the work calculated is negative, a pump is required; if it is positive, a turbine could be installed to extract energy from the system. The head required from the pump: ∆ ∆ ∆ The pump shaft power is calculated by taking the efficiency of the pump into account. The efficiency depends on the operating conditions and the type of pump. As a first estimate, efficiency could be taken as 90 % for reciprocating pumps. Figure 20.14 in the prescribed textbook provides estimates based on the head required and the flow rate of the liquid pumped. 6 100 Where: m = mass flow rate, kg/s ηp = pump efficiency, % Work delivered by a hydraulic recovery turbine is: Where ηt = turbine efficiency Example 5.1 Brine of S.G. 1.18 is to be pumped at 110 kg/min through 5 cm piping in the system shown here. The friction term is calculated as 0.418 kJ/kg. What is the power required to do the pumping? 2. 9m 1. 1m Consider the energy balance between the surface of the tank and the outlet of the pipe: ∆ ∆ ∆ 1180 9.8 1 9 418 110 60 910 Study example 20.6 in the prescribed textbook, Chemical engineering design. Activity 5.1 7 1. 2. 3. 4. 5. What is the purpose of using a pump? What is head in the context of pumping? Can work done by the pump be negative? What does this mean? What is the difference between a pump and a turbine? A pump takes water at 15 ˚C from a large reservoir and delivers it to the bottom of an open elevated tank 8 m above the reservoir surface through an 8 cm ID pipe. The inlet to the pump is located 3 m below the water surface, and the water level in the tank is constant at 11 m above the reservoir surface. The pump delivers 400 L/min. Assume a total equivalent length for the pipeline of 25 m. If the pump motor has an overall efficiency of 55%, determine the electrical power required to do the pumping. (Answer: W = 116 m2/s2) 5.3.4 Characteristic curves for centrifugal pumps Centrifugal pump performance is represented by multiple curves indicating either various impeller diameters at constant speed, or various speeds at constant impeller diameter The pump performance curve is characterised by plotting head against the flow rate as outlined in figure 20.15 in the prescribed textbook. Efficiency is plotted on the same curve. The following blog has a detailed explanation of how to read a pump curve: https://blog.craneengineering.net/how‐to‐ read‐a‐centrifugal‐pump‐curve. You could also watch the following video clip which has a useful explanation of pump curves: https://www.youtube.com/watch?v=s4pePBPhNU8 If the speed or impeller diameter of the pump changes, the resulting performance change can be calculated using affinity laws: The flow changes proportionally to the speed, i.e. if the speed doubles, flow will be doubled. The pressure changes by the square of the difference, i.e. if the speed doubles, pressure is multiplied by 4. The power changes by the cube of the difference, i.e. if the speed doubles, power is multiplied by 8. All types of pumps have operational limits. The best efficiency point is the operating point of highest efficiency and is also the point where the velocity and pressure are equal around the impeller. As the operating point moves away from the best efficiency point, velocity changes with pressure acting on the side of the impeller. The uneven pressure results in a radial thrust which results in excess load on the shaft bearings, excess deflection of the mechanical seal and uneven wear of the shaft. The following website explains the importance of locating the best efficiency point: http://www.maintenancetechnology.com/2013/05/the‐importance‐of‐best‐efficiency‐point‐bep/ If the pump is operated outside the recommended operating range, vortices in the pump could create cavitation, which is capable of destroying the pump casing and shortens the pump’s lifespan. Cavitation is defined as the formation of bubbles or vapour around the impeller. The vapour bubbles form as a result of low pressure due to the increased velocity at the inlet. A decrease in pressure results and if the pressure drops too low, lower than the vapour pressure, cavitation occurs. The 8 bubbles collapse, creating shock waves inside the pump and thus causing significant damage to the impeller. The following video clips show an animation of cavitation in a pump: https://www.youtube.com/watch?v=ZlrFMmGs_NI and https://www.youtube.com/watch?v=U‐ uUYCFDTrc The net positive suction head (NPSH) is related to how much suction lift the pump is capable of by creating partial vacuum. Atmospheric pressure then pushes the liquid into the pump. In other words, NPSH is the difference between the suction pressure and the vapour pressure. The pressure at the pump suction is above the vapour pressure of the liquid and is expressed as head of liquid. NPSH can be expressed in two parts: NPSHavailable = The absolute pressure at the suction port of the pump. This is a function of the system and must be calculated. NPSHrequired = The minimum pressure at the suction port of the pump to prevent cavitation. This is a function of the pump and needs to be supplied by the pump manufacturer. NPSHavailable > NPSHrequired to prevent cavitation. The formula for calculating NPSHavailable is as follows: Where: NPSHavailable = net positive suction head available at pump suction, m P = pressure above the liquid in the feed vessel, N/m2 z = height of liquid above the pump suction, m Pf = pressure loss in the suction piping, N/m2 Pv = vapour pressure of the liquid at pump suction, N/m2 ρ = density of the liquid, kg/m3 g = acceleration due to gravity, m/s2 Try watching the following clips for a better understanding of NPSH: https://www.youtube.com/watch?v=fCN_GnIpsEI and https://www.youtube.com/watch?v=55o3LcemAUg Turton et al (2009) suggest the following methods to increase the available NPSH: Decrease the temperature of the liquid at the pump inlet, which in turn will decrease the value of the vapour pressure. Increase the elevation of the pump, which increases the static head. 9 By increasing the diameter of the suction line, velocity is reduced, which lowers the frictional loss term. Example 5.2 A pump feeds toluene from a feed tank kept at atmospheric pressure and 55 ˚C. The pump is located at 2 m below the liquid level of the tank and there is 6 m of equivalent pipe length between the tank and the pump. The suction line is manufactured from commercial steel which has a roughness factor of 0.001 and an inside diameter of 0.02664 m. The flow rate of toluene is 10 000 kg/hr. Determine whether this is a suitable pumping arrangement if the NPSHrequired is 4.7 m. The following data is available for toluene: ∗ Where 4.1 . 10.97 ∗ is the vapour pressure of the fluid 10 . 870 At 55 ˚C the vapour pressure = 0.172 bar Velocity = 5.73 m/s therefore Re = 426 000, hence f = 0.005 4 2 and 1.10325 870 10 9.8 2 2 0.005 6 5.73 0.02664 11.88 9.8 2.00 2.01 0.172 870 10 9.8 7.57 4.30 The available NPSH is less than the required NPSH; therefore the pump arrangement is not suitable. 10 Study example 20.7 in the prescribed textbook, Chemical engineering design. You could also watch an additional example here: https://www.youtube.com/watch?v=SDJ10FJufCM Activity 5.2 1. Study example 5.2. Suggest ways to avoid cavitation. (Feedback: One method would be to increase the height of the liquid in the tank to 3 m. Calculate the available NPSH for this height and share the answer with your colleagues on the discussion forum.) 2. Do you think positive displacement pumps suffer from cavitation? Explain your reasoning. 5.3.5 System curve A system curve is a graphical representation of the mechanical energy equation. It consists of a plot of the total pressure head versus the liquid flow rate for a particular system. The correct pump is selected by combining the system curve and the pump curve: the operating point is where the system curve and the pump curve intersect. We discussed calculating the power requirements for a pump at a given flow rate in section 5.3.3. From the pump characteristic curve, we then read off the head available for the pump at the particular flow rate. Similarly, we could calculate a head available for various flow rate values through a system and whether the pump will be suitable. If we plot these values as a system curve, showing the W (work) required at various flows on the same axis as the pump characteristic curve, the intersection will correspond to the flow that will be obtained (see figure 5.4). Figure 5.4: Determination of flow (system curve) We illustrate the use of a system curve by means of an example. 11 Example 5.3 Water is to be pumped in this system: 2m 7m 1m 1.5 m Table 1 gives the head loss due to friction in the pipeline at various flow rates. Table 2 gives the Q‐H data for a 10 cm diameter pump running at 3 000 rpm. Determine the flow rate that will be obtained using this pump. 3 Q m /s 0.02 0.08 0.12 0.16 Table 1 Head loss m 6 25 57 87 Table 2 Data for 10 cm pump @ 3 000 rpm Q m3/s Head m 0.0 75 0.01 72 0.02 66 0.04 54 0.06 36 0.08 6 ∆ ∆ ∆ ∆ ∆ 10 Q m3/s 0.02 0.08 0.12 0.16 Head m 16 35 67 97 12 80 70 60 Head [m] 50 40 30 20 10 0 0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 0.16 0.18 Flow rate [m3 /s] Study example 20.8 in the prescribed textbook, Chemical engineering design. Activity 5.3 1. Water is to be pumped at 140 L/min through the system shown: 2. 0.5 m 4m 0.2 m 2m 1. 1m 0.55 m The pipeline is 3 cm ID and the roughness of the pipe material is 5 10 m. The actual length of the piping is 23 m and the equivalent length of an elbow is 30D. Other dimensions are given in the sketch. Determine the power consumption of the motor driving the pump if the overall efficiency is set at 65%. (Answer: W = 153 J/kg and power = 549 W) 2. Water is to be pumped from tank 1 to tank 2 using the system shown here. The table gives the loss due to friction in a 3 cm pipeline at various flow rates. You are supplied with a pump characteristic curve at 1 479 rpm. What flow rate would be obtained using this pump with a 150 mm impeller diameter? What power would the motor need to deliver? (Answer: Q = 8.8 m3/hr, head = 5.3 m, power = 0.28 kW) 13 Q (m3/hr) 2 4 6 8 10 12 14 Pf (m2/s2) 4.6 7.6 11.9 17.6 26.6 33.0 48.6 2. 0.5 m 3m 0.2 m 2m 1. 0.5 m 14 1 479 RPM pump curve 15 5.3.6 Pipe size selection Optimum pipe size denotes the best pipe size. From a simplistic standpoint, the best pipe size is the smallest size that will accommodate the application at hand. From a realistic standpoint, optimum pipe size can mean economically efficient over the life of a system. To estimate pipe sizes, you need to understand the velocity that fluid will have in the pipe. In section 20.10 in the prescribed textbook pipe velocities and pressure drops are discussed, with methods by different authors. For example, Rase (1953) relates the velocity of fluid through a pipe to the internal diameter of the pipe: Pump discharge: 0.06 Pump suction: 0.02 Steam or vapour: 0.2 0.4 0.1 / / / When considering pipe diameters, a pipe diameter is chosen based on the lowest annualised cost. The cost of the pipe increases with increasing diameter whereas the pumping costs decrease with increasing pipe diameter. The economic pipe diameter is calculated as follows: , 3.2 . Where: = mass flow rate, kg/s = density, kg/m3 = pipe inside diameter, m 5.4 Expanders and compressors Reading: To complete this section, study section 20.6 in your prescribed textbook, Chemical engineering design. 5.4.1 Types of compressors A compressor is a device used to increase the pressure of a gas by decreasing the volume. In this way, gas is transported through a pipe. When choosing a compressor, it is important to know the flow rate required, the differential pressure required and the operating pressure of the system. The compressor type could be broadly classified into two types: positive displacement includes reciprocating‐type compressors as well as rotary compressors dynamic compressors include centrifugal and axial compressors The principal types of compressors used in industry are reciprocating, centrifugal and axial flow compressors. 16 5.4.1.1 Reciprocating compressor This type of compressor is a positive displacement compressor that uses pistons driven by a crankshaft. A reciprocating compressor is driven by an electrical motor and could be a single‐stage or multistage type of compressor. Reciprocating compressors are used in a variety of applications, including domestic fridges. The following clip shows an animation of a reciprocating compressor in operation: https://www.youtube.com/watch?v=4ogkke0‐mXM 5.4.1.2 Centrifugal compressor A centrifugal compressor uses an impeller or rotating disk to force the gas to the rim of the impeller. The increase in kinetic energy results in an increase in pressure. Advantages of centrifugal compressors include high flow capacity, extreme reliability and low maintenance. Look at a 3D animation of a multistage compressor to visualise the operation of a centrifugal compressor: https://www.youtube.com/watch?v=leosVYDGb‐Q https://www.youtube.com/watch?v=s‐bbAoxZmBg You could also read more about the different types of centrifugal compressors at the following link: http://petrowiki.org/Centrifugal_compressor 5.4.1.3 Axial flow compressor An axial compressor uses an array of fan‐like foils to continuously pressurise gases. The fan‐like foils rotate continuously to accelerate the fluid which flows parallel to the axis of rotation, or axially. They are typically driven by an electric motor or a steam or a gas turbine and are multistage due to the nature of operation. Axial flow compressors are high efficiency compressors with the main advantage being high flow rate tolerance and a compact design. The following clip shows an animation of an axial flow compressor in operation: https://www.youtube.com/watch?v=KmG97bt41IU An economic operation of processes involving compression and expansion of large quantities of gases, such as ammonia synthesis, nitric acid production and air separation, depends on the efficient recovery of the energy of compression. Often, energy recovered by expansion is used to drive the compressors directly. If a gas is found to contain condensable components, it may be advisable to consider heating the gas by heat exchange with a higher temperature process stream before expansion. The gas can then be expanded to a lower pressure without condensation and the power generated increased. The energy recovered from the expansion of a gas can be estimated by assuming polytropic expansion. In the process industries, a turbo‐expander or expansion turbine is used which is a centrifugal or axial flow turbine through which a gas at a high pressure is expanded to produce work that could be used to drive a compressor or generator. 5.4.2 Compression of gases The work done in compressing or expanding a gas is given by: 17 For isothermal (constant temperature) operation The work done is therefore: Where: = initial pressure = final pressure = initial volume R = universal gas constant M = molecular weight of the gas Commercial expanders and compressors are polytropic. The polytropic process may be defined as a and is process where the relationship between P and V may be expressed by the polytropic expansion coefficient. The work is therefore defined as: 1 1 1 1 Where: Z = compressibility factor (equal to 1 for an ideal gas) ‐ the compressibility chart shown in figure 20.10 in the prescribed textbook R = universal gas constant, 8.314 J/K.mol T1 = inlet temperature, K W = work done, J/kg 5.4.3 Polytropic compression and expansion Reading: To complete studying this section, revise your thermodynamics module and make sure you understand the collection of data from steam tables and Mollier (enthalpy‐pressure‐temperature‐ entropy chart) diagrams. Section 20.6.2 in the prescribed textbook has a revision section on Mollier diagrams. Study example 20.2 in the prescribed textbook. 18 At conditions away from the critical point: 1 1 Where: 1 1 is the polytropic efficiency defined as: : : You can see an estimate for the polytropic efficiency in figure 20.8 in the prescribed textbook. Study example 20.3 in the prescribed textbook. Example 5.4 O2 is contained in a piston cylinder device at the following conditions: (P1 = 600 kPa, T1 = 200°C, V1 = 0.02 m3). The gas then expands according to the equation PV1.2 = constant to a final temperature T2 = 100°C. Determine a) the final volume b) the work done Before proceeding with the problem, convert the temperatures to Kelvin. This means that T1 = 473 K and T2 = 373 K. Remember that when solving a problem for a polytropic process, the equations as applied to adiabatic processes may be used, substituting δ for γ (given above, PVγ = constant therefore γ = 1.2). Determine V2. T1 and T2 are given. Starting with the equation to obtain , you can rearrange . Substituting values into this equation: 473 373 . 0.02 19 0.0656 The work done may then be determined 1 1 . This modification can be made since Solving for W. 8.314 . 1.2 473 373 473 1 1 4157 Example 5.5 1 kmol of air has an initial temperature of 260 °C and a volume of 3 m3. The gas is expanded at constant pressure to 9 m3. After this a polytropic expansion process occurs according to the equation PV1.5 = const. The cycle is then completed by an isothermal compression process. All processes are reversible. Determine the amount of heat received and rejected during the cycle, assuming air to be an ideal gas with γ = 1.4 and R = 8.314 kJ.kmol‐1.K‐1. Consider the constant pressure process to occur between points 1 and 2, the polytropic process between points 2 and 3 and the isothermal process between points 3 and 1. Constant pressure process 1‐2 Before proceeding further, determine initial pressure. 8.314 260 . 273 3 1477 The temperature at the end of the constant pressure process can now be determined from 9 3 533 1599 1.4 and CP – CV = R. Resolving these CP can be determined from the equations simultaneously gives a value for CP of 29.1 kJ.kmol‐1.K‐1 and subsequently a value for CV of 20.785 kJ.kmol‐1.K‐1. For a constant pressure process ∆ 31020 ∆ 29.1 . 1599 533 . 20 Since the system contains 1 kmol, Q = 31 020 kJ supplied to the system. Polytropic process 2‐3 From the problem statement, you can see that T3 = T1 = 533 K. Q will be determined from ∆ ∆ Determination of ΔU is straightforward. ∆ 20.785 533 . 1599 22156.8 Determination of W is somewhat more complicated since P varies during the process. However, using the polytropic equation PV1.5 = constant the integral may be solved. The equation PV1.5 = P2V21.5 = k may be rearranged to the form . where k = P2V21.5. This may then be substituted into the work integral so that . 0.5 . To fully evaluate the above integral, a value for V3 must be found which is done by using . 1599 533 9 81 therefore . 1 1 1477.1 0.5 9 0.5 . 1 √81 1 √9 17725.2 Q = ΔU – W = ‐ 22156.8 – (‐17725) = ‐ 4431.35 kJ Isothermal process 3‐1 21 ln 8.314 . 533 ln 3 81 14605 Heat amounting to 31 020 kJ is supplied to the system during the first process. Heat amounting to 19 036 kJ is removed during the polytropic and isothermal processes. The net effect is that the system has a heat gain of 11 983.6 kJ over the cycle. Study examples 20.3 and 20.4 in the prescribed textbook, Chemical engineering design. 5.4.4 Multistage compression To achieve high pressure ratios when undertaking compression of a gas, a multistage compressor is needed. Multistaging is simply the compression of air or gas in two or more cylinders in place of a single cylinder compressor. It is often used in reciprocating compressors when pressure of 300 KPa and above is desired, in order to save power, limit the gas discharge temperature and pressure differential per cylinder as well as prevent vaporisation of lubricating oil and ignition if the temperature becomes too high. It is common practice for multistaging to cool the air or gas between stages of compression in an intercooler. The purpose of intercooling is to reduce the temperature of the gas, which reduces its volume so that the work done by the compressor will be less for the reduced volume, which will reduce the input power. Stages are normally selected to give equal work in each stage. For a two‐stage compressor the interstage pressure ( ) is given as: Study example 20.5 in the prescribed textbook. Activity 5.4 1. List the types of compressors and give an example of each. 2. What is the significance of the compressibility factor Z? 3. A colleague argues that oxygen gas at 160 K and 3 MPa can be treated as an ideal gas with a percentage error of less than 10%. Is the argument valid? 4. Nitrogen (2 kg) is contained in a frictionless piston‐cylinder system at 300 K and 100 kPa. . until a temperature of 360 K is Compression occurs according to the relationship reached. Calculate the work done. (Answer: 89 kJ) 5. Redo example 5.5 assuming the processes are carried out irreversibly with an efficiency of 80% for each step. 6. Do problem 20.4 in the prescribed textbook, Chemical engineering design. 7. Are your mathematical knowledge and skills sufficiently developed to follow the equations you encountered in this unit? If not, you may need to go back to some of your mathematics 22 and thermodynamics modules and revise the relevant aspects. Also remember that you could ask your lecturer or e‐tutor for help with any aspects you might not understand. 5.5 Valves Reading: To complete this section, study sections 20.5 and 20.11 in your prescribed textbook, Chemical engineering design. 5.5.1 Types of valves A valve can be defined as a device that aids in directing and/or controlling the flow of fluid by opening or closing. Flow could also be regulated by a movable part that partially obstructs the passageway. An open valve will allow the flow of fluid from a region of higher pressure to lower pressure. Two main classes of valves are shutoff valves, which allow for closing off the flow control valves, which are used to regulate the flow For shutoff valves, the most commonly used valves to close off fluid flow in a pipe are gate, ball and plug valves. These types of valves are available commercially in a wide range of sizes and allow for manual or automatic operation. For operations where quick open and close action is required, plug or ball valves are employed rather than gate valves. Operations requiring flow control employ the use of globe or diaphragm valves as these give a smooth control of flow when transitioning between closed and open. Butterfly valves are used when gas and vapour flows require automated control. Figure 20.6 in the prescribed book shows the design schematic for different types of valves. In the following clip the basic types of valves used in industry and their operation are discussed: https://www.youtube.com/watch?v=W5gC‐y7aEO8 5.5.2 Sizing of valves A control valve performs the task of allowing for the control of fluids so that a control variable such as fluid pressure, temperature or level could be controlled. In addition to controlling the flow, it may be used to shut off flow completely. It is, however, seldom fully open or closed but rather in an intermediate position controlling the fluid flow through a valve. As such, it needs to be able to withstand the erosive effects of the flowing fluid while at the same time maintaining accurate positioning to prevent deviation from the set process variable. To maintain the flow correctly, it would be important to correctly size the control valve. Based on the Bernoulli equation, the valve sizing equation was developed as follows: ∆ 23 Where: = capacity in gallons per minute = valve sizing coefficient determined experimentally for each valve type ∆ = pressure difference in psi = specific gravity of the fluid The equation use is limited to non‐compressible, non‐choking turbulent liquid flow. For gases, manufacturers would need to be consulted. To calculate the expected for a valve controlling water or liquids that behave like water, we can calculate the valve sizing coefficient as follows: ∆ It is important to note that the values have been based on water, and viscosity correction factors would need to be applied for fluids deviating from water properties. A graph of the Reynolds number plotted against a correction factor ( ) is used to determine the correction factor needed. The actual is then calculated: , The graph is normally provided from the manufacturer. Pressure drop in a valve is created by the system demand and not the valve alone. It is a critical element in valve sizing to ensure proper valve selection. Pressure drop is directly proportional to the flow rate, i.e. the higher the flow rate, the higher the pressure drop and vice versa. The pressure drop for liquid flow through a valve could be predicted from the equation: ∆ A rule of thumb is that flow rate should normally be sized for a higher flow rate, i.e. 1.3 1.1 The rangeability of a control valve is defined as the ratio between the maximum controllable flow and the minimum controllable flow. Rangeability of a valve is influenced by valve design, actuator and positioner. Rangeability values normally vary between 20 and 50. The turndown ratio of a valve is defined as the ratio between the normal maximum controllable flow and the minimum controllable flow. Turndown ratios are usually 70% of the rangeability value. Example 5.6 24 A high integrity air‐actuated valve is needed in a chromatograph system analysing different types of gasoline ( for gasoline is 0.71). The flow required is 14.5 GPM and the inlet pressure is 375 psig. The outlet pressure has been determined as 25 psig. The valve selected from a manufacturer’s of 0.93. Will this valve be appropriate for the chromatograph analyser? brochure has a ∆ 14.5 0.71 375 25 0.65 The calculated is less than the available ; therefore the valve will be adequate. Study example 20.13 in the prescribed textbook, Chemical engineering design. Activity 5.5 1. An engineer requires a shutoff valve with a flow rate of approximately 10 GPM, and flow medium is clean water at room temperature. The inlet pressure is determined as 3 000 psig and the outlet pressure is at 2 900 psig. The valve she has selected has a of 1.20. ( Calculate if the valve selected will provide the required flow rate. (Answer: Q = 12 GPM) 0.32) used in an ammonia system with an 2. Calculate the pressure drop for a check valve ( of 0.662. The flow rate of the fluid is 2.25 GPM ammonia. (Answer: 33 psig) 5.6 Summary Having completed this learning unit, you should be able to select the appropriate type of pump and specify the necessary design data for the pump such as flow rates, power requirements and efficiency. Calculations around work done by compression and expansion should also be possible and you now know the necessary information to calculate the number of stages in a compressor. You learnt about different types of valves and calculations in computing valve size. This learning unit has provided you with important information in order to understand the different types of equipment that may be used to achieve the transport of liquids and gases in the process. Sources consulted Centrifugal pump. (n.d.). https://commons.wikimedia.org/wiki/File:Centrifugal_Pump_en.svg [Accessed 28 September 2017]. Centrifugal pump. (n.d.). https://commons.wikimedia.org/wiki/File:Centrifugal_pump_volute_Richards_1894.png [Accessed 28 September 2017]. 25 Gear pump. (n.d.). https://en.wikipedia.org/wiki/Pump#/media/File:Gear_pump.png [Accessed 28 September 2017]. Towler, G & Sinnott, RK. 2013. Chemical engineering design: principles, practice and economics of plant and process design. 2nd edition. Oxford: Butterworth‐Heinemann. Turton, R, Bailie, RC, Whiting, WB & Shaeiwitz, JA. 2009. Analysis, synthesis and design of chemical processes. 3rd edition. New Jersey: Prentice Hall. 26 Learning unit 6: Economic analysis of processes 6.1 Introduction One of the main objectives of any chemical process is to make a profit. Therefore, an estimate of the investment required to design, construct and start up the entire plant, as well as the cost associated with each manufacturing step is needed to determine the overall profitability of the process. The design engineer must be able to make cost estimates to choose between various design alternatives and to optimise the design. In this learning unit we review the components that make up the capital cost of a plant and the components of the operating costs. We discuss costing techniques, which can be used to make preliminary estimates of capital and operating costs at the flow sheet stage. You will learn techniques to assess economic viability and the criteria used to compare design alternatives in this learning unit. 6.2 Learning outcomes At the end of this learning unit, you should be able to estimate the cost of major process equipment use the detailed factorial method to estimate the capital cost of a process evaluate the production costs (operating costs) and revenue of a project assess the economic viability of a project using various economic criteria 6.3 Capital cost estimating Reading: To complete this unit, study chapter 7 in your prescribed textbook, Chemical engineering design. 6.3.1 Capital investment The total capital investment (TCI) is the capital required for the design, construction and start‐up of a new plant or a retrofit of an existing plant. The TCI consists of fixed capital investment (FCI) and working capital. 6.3.1.1 Fixed capital investment The FCI represents the capital required for designing, constructing and installing the plant and expenses incurred to prepare the plant site. It is the depreciable portion of the TCI, i.e. it is only recovered as scrap value. The FCI is made up of (Towler & Sinnott 2013) the inside battery limits (ISBL) investment—the cost of the major process equipment the modifications and improvements that must be made to the site infrastructure, known as offsite or the outside battery limits (OSBL) investment engineering and construction costs contingency charges The ISBL plant cost comprises the cost of procuring and installing all the process equipment. This would include major process equipment bulk items such as piping and valves civil works and labour costs 1 Towler and Sinnott (2013) give a detailed list of these costs. Offsite cost or OSBL investment comprises the capital required for developing the site’s infrastructure, which is crucial for the operation of the plant. These include utilities and auxiliary facilities. Offsite investments are listed in Towler and Sinnott (2013). For preliminary estimates, offsite costs are typically estimated as a percentage of ISBL costs. The offsite costs are usually between 20% and 50% of ISBL cost for petrochemical processes, and 40% is typically used as an initial estimate when the site details are not known (Towler & Sinnott 2013). For grassroots plants, where no utilities and infrastructure are available, the offsite costs tend to be much higher than for existing sites which have well‐ developed infrastructure. The engineering costs (or contractor’s charges) comprise the costs of design and other engineering services necessary to implement the project. Most companies hire the services of one or more of the major engineering contracting firms to design the projects. Examples of these firms are Foster‐Wheeler, Fluor and KBR. Engineering costs are not directly proportional to the size of the project and vary depending on the market demand for engineering services, timelines and project scope. A rule of thumb for engineering costs is 30% of ISBL plus OSBL cost for smaller projects and 10% of ISBL plus OSBL cost for larger projects as discussed in the prescribed textbook. There is always uncertainty in estimating the precise cost of a project, especially since the final installed cost of most pieces of equipment is only known when the installation has been completed. Furthermore, changes in the scope of a project, fluctuations in currency exchange rates or changes in metal prices, as well as various other unpredictable events add to the uncertainty of the cost estimate. A contingency charge refers to the amount added to the project budget to allow for this type of uncertainty. A minimum contingency charge of 10% of ISBL plus OSBL cost should be used on all projects. Higher contingency charges (up to 50%) can be included, especially if new and unproven technology is being employed. 6.3.1.2 Working capital Working capital may be defined as a measure of a company’s efficiency and short‐term financial status. Working capital is the additional money required for the operation of the plant, especially during the initial stages of operation. It is fully recoverable and is therefore not depreciated. Working capital includes the money invested in raw material inventory – usually estimated as 2 weeks’ to 1 month’s delivered cost of raw materials product and by‐product inventory – estimated as 2 weeks’ to 1 month’s cost of production cash on hand – estimated as 1 month’s operating expenses such as salaries, payment for raw materials, services accounts receivable – products shipped but not yet paid for, estimated as 1 month’s cost of production credit for accounts payable – feedstocks, solvents, catalysts, packaging, etc. received but not yet paid for, estimated as 1 month’s delivered cost spare parts inventory – estimated as 1% to 2% of ISBL plus OSBL investment cost Working capital can be estimated as a percentage of the FCI or a percentage of the annual sales. In terms of the FCI, the working capital can vary between 5% and 30% of the fixed capital cost, depending on the type of product being produced. A typical figure for petrochemical plants is 15% of the fixed capital (ISBL plus OSBL cost) (Towler & Sinnott 2013). In terms of annual sales, percentage values vary from 15 to 49% with 30 to 35% being a reasonable value. Working capital can be better estimated by calculating the inventory costs. 2 Activity 6.1 1. Differentiate between fixed and working capital. 2. How is the total capital investment calculated? 3. Differentiate between direct field costs, indirect field costs and offsites. Give examples of each. 6.3.2 Classes of capital cost estimates Various types of estimates of the capital investment of a process can be prepared. These different estimates can be distinguished from one another by the following five characteristics (Dysert 2003): degree of project definition end use of estimate estimating methodology estimating accuracy time and effort to prepare estimate Typically, capital cost estimates can be classified into five classes (Towler & Sinnott 2013): 1. Class 5 estimate (ratio estimate, order‐of‐magnitude estimate) The accuracy of this estimate ranges from +30‐50%. This is based on costs of similar projects and does not require design information. This estimate is used mainly for screening purposes. 2. Class 4 estimate (study estimate, feasibility estimate) The accuracy of this estimate is + 30%. It is used to choose between design alternatives and it is based on knowledge of major items of equipment. 3. Class 3 estimate (budget authorisation estimate, scope estimate) The accuracy of this estimate is +10‐15%. It is based on a more accurate sizing of major equipment and layout of the equipment, as well as an estimate of utilities and an estimate of the piping and instrumentation. This estimate is used to approve funding for the design. 4. Class 2 estimate (project control estimate, definitive estimate) The accuracy of this estimate is +5‐10%. It is based on data that is almost complete. The estimate is based on a preliminary specification of all equipment, utilities, piping and instruments, electrical and offsites. 5. Class 1 estimate (check estimate, contractor’s estimate) The accuracy of this estimate is ±5%. This is based on complete engineering drawings of the process as well as the utilities and offsites and definite quotes for all expensive and long‐lead items from vendors (suppliers of items). This estimate is used in the construction of the plant. The relative cost required to prepare an estimate of a class 5 estimate to a class 1 estimate starts at 1 and can go to as much as 100 ( Turton et al 2009). For example, if a class 5 estimate costs 0.015% of the project cost, then a class 1 estimate would be 1.5% of the project cost. 6.3.3 Order‐of‐magnitude estimates The simplest method of estimating the cost of a plant when cost data is not available is to utilise previous cost data of a similar plant. The scaling is done based on the capacity of the plant. 3 The capital cost of a plant is given by: Eq. 0.1 Where: ISBL capital cost of the plant with capacity ISBL capital cost of the plant with capacity The exponent n is typically 0.8 to 0.9 for processes that use a lot of mechanical work or gas compression (e.g. methanol, paper pulping, solids‐handling plants). For typical petrochemical processes, n is usually about 0.7. For small‐scale, highly instrumented processes n is in the range 0.4 to 0.5. Averaged across the whole chemical industry, n is about 0.6, and hence equation 6.1 is commonly referred to as the ‘‘six‐tenths rule’’. This value can be used to get a rough estimate of the capital cost if sufficient data is not available to calculate the index for the particular process. 6.3.4 Cost escalation Cost escalation is defined as the changes in the price of goods or services rendered during a specific period. The method of estimating cost escalation is to use historical data. This is presented as forecasts of construction costs of the proposed plant as well as estimates of equipment costs. Eq. 0.2 It is advisable to divide each job into its separate components and indices to obtain an accurate estimate. Composite indices are also available in published trade journals such as Chemical Engineering Oil and Gas Journal Engineering News Record Study examples 7.4 and 7.5 in the prescribed textbook. The standard indices available are usually restricted to a particular region such as the US Gulf Coast Basis (USGC) and Northwest Europe Basis (NWE). Unfortunately, there are no South African or African‐based indices. The index values cannot be used to get accurate results in other regions of the world. This variation might be due to various factors such as materials cost and availability labour cost and availability equipment and labour costs including transportation costs import duties and local taxes currency exchange rates To account for these fluctuations, a location factor can be used (Towler & Sinnott 2013): Eq. 0.3 = location factor for location A relative to USGC basis and can be found in table 7.7 in the prescribed Where textbook. Study example 7.6 in the prescribed textbook. 4 Example 6.1 A fertiliser factory in Sasolburg with a capacity of 3 000 tons/yr was destroyed by a fire. The plant was originally built in 2009 at a cost of R8 000 000. A new plant will be erected during 2016. This plant will have a capacity of 6 500 tons/yr. The scaling factor for this type of plant is 0.65. Cost indices 2009 = 521.9 2016 = 541.7 Calculate the cost of the plant in 2016. Firstly, we use the cost escalation equation (Eq. 6.2) to update the initial cost to 2016. To build a 3 000 tons/yr plant in 2016 it would cost: 2016 2016 541.7 521.9 8 000 000 8 303 506 We then use the Eq 6.1 to scale up to the required capacity. To build a 6 500 ton/yr plant in 2016 it would cost: 8 303 506 6 500 3 000 . 13 725 444 Activity 6.2 1. Explain the concept of economy of scale. 2. When would you use a cost exponent n = 0.6? 3. A gas‐to‐liquids plant producing 38 000 bbl/day of product is estimated to cost $3.2 billion. What would be the cost of a 96 000 bbl/day plant? Use the six‐tenth rule. 96 000 . $3.2 $5.6 38 000 6.3.5 Estimating purchased equipment costs Purchase equipment cost is normally correlated from similar recent equipment purchases that may have been made for projects. These are normally done by cost engineering specialists in order to ensure reliability and experienced judgements. Otherwise, a quotation can be obtained from various vendors for each piece of equipment. Additionally, these equipment costs can be estimated using open literature or websites such as www.equipnet.com or http://www.matche.com/equipcost/Default.html or software such as Aspen ICARUS technology. Where reliable data for costing is not available, equation 6.1 or the following correlation can be used: Eq. 0.4 Where: = purchased equipment cost on a US Gulf Coast basis, January 2010 , = cost constants available in table 7.2 in the prescribed textbook 5 = size parameter, lower and upper limits given in table 7.2 in the prescribed textbook = exponent for a particular type of equipment Cost charts have also been used to estimate the cost of equipment. These can be found in older versions of the prescribed book and other chemical engineering design textbooks. However, it is much more convenient to use correlations such as Eq. 0.4Eq. 0.4Eq. 6.4. For specialised equipment such as reactors, heat exchangers and distillation, we recommend that detailed methods of cost estimating be used as opposed to the shortcut estimation method. The detailed estimate takes into account the required parts, fabrication of the equipment, machinery involved and the labour needed for each of the steps. An example of the cost breakdown structure for a shell and tube heat exchanger is provided in table 7.3 in the prescribed textbook. 6.3.6 Shortcut method of sizing equipment The cost of process equipment is usually related to a size parameter. For example, the cost of a heat exchanger can be related to its area. It is useful to have shortcut methods of sizing equipment. These have been developed over a number of years and are often formed through the experience of the design engineer as well as texts they may consult. This eliminates the need for extensive calculations to calculate equipment sizes, operating conditions and equipment performance in the preliminary design stages. They may involve setting temperatures, pressures, excess amounts of chemicals etc. The engineer should not shy away from using rules of thumb, nor rely blindly upon them. Often we may find that one heuristic contradicts another and while it can reduce time in solving a problem, its acceptance will depend on the immediate context rather than taken as an absolute standard. Tables of heuristics for a number of design topics are provided on this web link: http://people.clarkson.edu/~wwilcox/Design/heurist.pdf Now we briefly discuss shortcut methods for sizing of major equipment. Shortcut for vessel sizing 5 min 1. Select the volume for hold‐up: 2. 2 where 3. Select Length = 4*Diameter: is the liquid mass flow rate 4 4. 5. If D ≤ 1.2 m vessel is placed vertically, if not, place vessel horizontally 6. Pressure (rated) = 1.5 pressure (actual) Shortcut for reactor sizing 1. First step in the preliminary design, therefore no kinetic model will be available. 2. Mass balance based on product distribution, thus there will be a major influence on the final cost. 3. Assume the reactor equivalent to laboratory reactor, adiabatic reactors are isothermal at average temperature. 4. Assume a space velocity and calculate the volume: 1 1 6 Where = flow rate = molar density = volume of catalyst = void fraction of catalyst Heat transfer equipment sizing 1. ∆ 2. is obtained from the energy balance 3. U is estimated depending on the configuration and media used 4. A is the area – if phase changes, use 2 heat exchanges, therefore A = A1 + A2 log mean temperature, see learning unit 3 5. ∆ Shortcut for compressors or turbine sizing 1. Centrifugal compressors are the most commonly used compressors giving high capacity:low compression ratios. 2. Reciprocating compressors give low capacity:high compression ratios. 3. Assumptions: ideal gas behaviour, isentropic and adiabatic behaviour. 0.9 and 0.8 4. Electric motor driving compressor: Brake horsepower 1.39 and 0.8 and 1.562 5. Turbine driving compressor Maximum horsepower compressor = 10 000 hp = 7.5 MW Maximum compression ratio = 5 6. Staged compressors ‐ decrease work using intercoolers in N stages, work is minimised when compression ratios are the same: / ⋯……… / Rules of thumb = 2.5 = N Shortcut for pump sizing 1. Centrifugal pumps are the most common; assume isothermal conditions 2. Brake horsepower: 3. = 0.5 and = 0.9 Example 6.2 A pump is used to feed toluene to a process. The information available for this pump is as follows: Flow rate of toluene = 13 300 kg/hr Density of toluene = 870 kg/m3 Temperature in = 55 ˚C 7 Pressure in = 1.2 bar Pressure out = 25.8 bar Efficiency for a reciprocating pump = 0.75 Consult the table of heuristics on this web link: http://people.clarkson.edu/~wwilcox/Design/heurist.pdf For pumps: Rule 1: Power for pumping liquids: HP = (gpm)(psi difference)/(1 714) (fractional efficiency) ∆P = 25.8 bar‐1.2bar = 24.6 bar = 357 psi (Rules 4 to 7 describe the type of pump based on the flow rate and head.) Volumetric flow rate = gpm = (13 300 kg m^3)/(hr 870 kg) hr/(60 min) (264 gallons)/m^3 = 67.3 Therefore HP = (67.3 × 357)/(1 714 × 0.75) = 18.7 hp (14 kW) To size the pump using equipment cost constants from table 7.2 in the prescribed textbook, we need the size in terms of L/s: 13 300 870 1 000 3 600 4.2 / Choose a single‐stage centrifugal pump. 8 000 240 4.2 . $8,873.24 Study example 7.2 in the prescribed textbook. 6.3.7 Preliminary and study estimates For preliminary and study estimates, the capital cost for chemical process plants can be estimated based on the purchase cost of the major equipment required for the process. The other costs (direct and indirect costs) are estimated as a factor of the equipment cost. Typically, these estimates have an accuracy of 20‐30%. The accuracy of a cost estimate will reflect the stage of the design as well as the available data at the time of the calculation. 6.3.7.1 Lang factor The ISBL capital cost is given as a function of the total purchased equipment cost by the Lang equation: Eq. 0.5 Where: = total ISBL capital cost (includes engineering costs) ∑ = total delivered cost of the major equipment = Lang factor or installation factor 8 The values for F as proposed by Lang are F = 3.1 for solids processing plant F = 4.74 for fluids processing plant F = 3.63 for a mixed solids/fluids processing plant 6.3.7.2 Individual factors for cost items Instead of using a single factor in the Lang equation, more detailed estimates use individual cost factors to incorporate direct cost items. Complete lists of these items are in the prescribed book. Typical factors for different direct cost items (such as piping and instrumentation, utilities, civil works and equipment erection) are given in table 6.1 as taken from Towler and Sinnott (2013). The ISBL cost is determined by multiplying the total purchased equipment cost by the appropriate factors. The indirect cost items (design and engineering costs, contractor’s fees, contingency) are included as a factor of the ISBL cost. Note that the installation factors provided in this table are for equipment manufactured from carbon steel only. Table 6.1: Typical factors for estimation of project fixed capital cost (carbon steel only) (Towler & Sinnott 2013) Process Type Item Fluids Fluids‐Solids Solids 0.3 0.5 0.6 Piping 0.8 0.6 0.2 Instrumentation and control 0.3 0.3 0.2 0.2 0.2 0.15 Civil 0.3 0.3 0.2 Structures and Buildings 0.2 0.2 0.1 Lagging and paint 0.1 0.1 0.05 3.3 3.2 2.5 Offsites (OS) 0.3 0.4 0.4 Design and Engineering (D&E) 0.3 0.25 0.2 Contingency (X) 0.1 0.1 0.1 Major Equipment total purchase cost Equipment erection Electrical ISBL cost, ∑ Total fixed capital cost = 1 1 & 9 = 1.82 1.89 1.82 =∑ 6.00 6.05 4.55 For other materials, Towler and Sinnott (2013) suggest a material factor, be applied: Eq. 0.6 The material factor is not merely a ratio of the two costs, but rather should be applied to the Lang equation and expanded to give the cost for each piece of equipment: Carbon steel basis, estimating the cost of alloy construction: ,, 1 Eq. 0.7 Alloy basis: ,, Where: ,, ,, 1 / Eq. 0.8 = purchased equipment cost of equipment i in carbon steel = purchased equipment cost of equipment i in alloy = total number of pieces of equipment = installation factor for piping = installation factor for equipment erection = installation factor for electrical work = installation factor for instrumentation and process control = installation factor for civil engineering work = installation factor for structures and buildings = installation factor for lagging, insulation and paint You can find values for typical engineering alloys in table 7.6 in the prescribed textbook. The following flow sheet (figure 6.1) summarises the procedure used in the factorial method to make an approximate estimate of the capital investment needed for the project: 10 Figure 6.1: Summary of the factorial method 6.3.7.3 Guthrie method Guthrie proposed the method of estimating equipment costs after an optimal design has been developed in order to improve the accuracy of the estimate by up to as much as 20% (Seider et al 2006). As with the Lang method, the free on board (fob) purchase cost of each piece of equipment must be estimated; however, the bare module cost estimate introduced by Guthrie uses individual factors for each piece of equipment. The Guthrie method also takes into account the different material of construction factors as well as operation for high pressure. The equation for total capital investment by the Guthrie method is 1.18 Eq. 0.9 Where: = total capital investment = total working capital = total permanent investment = total bare module cost – this is a summation of all items of process equipment, process machinery, spares, storage tanks and surge tanks The factor 1.18 covers a contingency of 15% and a contractor’s fee of 3%. The working capital can be estimated to be 15% of the TCI. Seider et al (2006) gives the method step‐by‐step as follows: 11 1. Prepare an equipment list with details of equipment title, label, size, material of construction and design temperatures and pressures. . and the corresponding cost index 2. Calculate equipment cost 3. Update the cost data to the current cost index. For each piece of equipment determine the Guthrie factors (table 6.2). Bare module cost is calculated using the equation 1 Eq. 0.10 Where: = bare module factor = equipment design factor = pressure factor = material factor ) cost by adding the bare module costs of each piece of equipment. 4. Obtain the total bare module ( 5. Obtain the total permanent investment and total working capital to obtain the total capital investment: 1.18 Eq. 0.11 This equation does not take into account plant start‐up. If this cost is known, it should be added to this equation. Table 6.2: Bare module factors of Guthrie for ordinary materials of construction and low to moderate pressures (Seider et al 2006) Bare module factor Furnaces and direct fired heaters, shop‐fabricated Furnaces and direct fired heaters, field‐fabricated Shell and tube heat exchangers Double pipe heat exchangers Fin tube air coolers Vertical pressure vessels Horizontal pressure vessels Pumps and drivers Gas compressors and drivers Centrifuges Horizontal conveyors Bucket conveyors Crushers Mills Crystallisers Dryers Evaporators Filters Flakers Screens 2.19 1.86 3.17 1.80 2.17 4.16 3.05 3.30 2.15 2.03 1.61 1.74 1.39 2.30 2.06 2.06 2.45 2.32 2.05 1.73 Activity 6.3 1. Differentiate between free on board (fob), delivered equipment cost and installed equipment cost. 12 2. Study example 7.3 in the prescribed textbook. In this example you are required to calculate the ISBL capital cost of the modification of the plant. It is important to note that in this example, you are first required to convert the units to those required for the correlations in table 7.2. As per table 7.2, units for size for a pressure vessel (which would include the distillation column) are shell mass measured in kg. 6.4 Cost of production Reading: To complete this unit, study chapter 8 in your prescribed textbook, Chemical engineering design. Besides the capital investment, the other important cost to consider is the cost of production. This covers the expenses for the running of the plant, after it has been built and commissioned. The production cost is a recurring cost, unlike the capital investment which is a once‐off cost. The total production costs of the project include all the manufacturing costs (also called operating costs or production costs), general overhead and the distribution and marketing of the product. The operating costs can either be calculated as an annual cost (e.g. R/year) or a cost based on a unit of production (e.g. R/ton or R/barrel). In most cases, an annual basis is preferred. The operating cost can be divided into two main components: (i) variable operating costs and (ii) fixed operating costs. The following video clip summarises costs of production: https://www.youtube.com/watch?v=ucJBO9UTmwo 6.4.1 Variable operating costs Variable operating costs depend on the plant capacity/output or operation rate. Examples of variable operating costs are (Towler & Sinnott 2013) 6.4.2 raw materials consumed by the process utilities—fuel burned in process heaters, steam, cooling water, electricity, raw water, instrument air, nitrogen and other services brought in from elsewhere on the site consumables—solvents, acids, bases, inert materials, corrosion inhibitors, additives, catalysts and adsorbents that require continuous or frequent replacement effluent disposal packaging and shipping—drums, bags, tankers, freight charges, etc. Fixed operating costs Fixed operating costs do not depend on the plant capacity/output or operation rate, i.e. they are unaffected by changes in the capacity of the plant. Fixed costs include the following (Towler & Sinnott 2013): Operating labour – these are normally shift positions. As an initial estimate in South Africa R300 000 per annum per shift position could be used. For the US Gulf Coast regions, estimates are normally $50,000 per shift position per annum. Supervision—usually taken as 25% of operating labour. Direct salary overhead—usually 40 to 60% of operating labour plus supervision. Maintenance, which includes both materials and labour, and is typically estimated as 3 to 5% of ISBL investment. Property taxes and insurance—typically 1 to 2% of ISBL fixed capital. 13 Rent of land (and/or buildings)—typically estimated as 1 to 2% of ISBL plus OSBL investment. Most projects assume that land is rented rather than purchased, but in some cases the land is bought and the cost is added to the FCI and recovered at the end of the plant life. General plant overhead—charges to cover corporate overhead functions such as human resources, research and development (R&D), information technology, finance, etc. Corporate overhead varies widely depending on the industry sector. Plant overhead is typically taken as 65% of total labour (including supervision and direct overhead) plus maintenance. Allocated environmental charges—typically 1% of ISBL plus OSBL cost. Running licence fees and royalty payments—i.e. those not capitalised at the start of the project. Capital charges—these include interest payments due on any debt or loans used to finance the project, but do not include expected returns on invested equity capital. Sales and marketing costs—in some cases these are considered part of general plant overhead. They can vary from almost zero for some commodities to millions of rand a year for branded items such as foods, toiletries, medication and cosmetics. Activity 6.4 1. Which item do you think is the main contributor to the overall production cost of a plant? 2. Where would you source pricing data for raw materials? What would you expect the units of the pricing data to be? 3. How would you determine the amount of raw material and utilities required in a plant? 4. Download and study the template which summarises the production cost of a plant from the prescribed book’s website: http://booksite.elsevier.com/Towler 6.4.3 Revenues Revenue is defined as the income a business generates from its normal operation. For a process industry, it would include sales of both main and by‐products. Often by‐products from a chemical process are sold for a minimum price in order to generate some income rather than incurring costs towards waste disposal. It is important to note that in some cases the by‐product could be converted to a more valuable product; therefore careful analysis of the economic viability should be carried out. The rule of thumb suggested by Towler and Sinnott (2013) is that for by‐ product recovery to be economically viable, the benefit needs to be greater than $200,000 per annum. The revenue (using an annual basis) is calculated from the unit price and the production rate of each product as follows: Eq. 0.12 The gross margin can be calculated from: Eq. 0.13 This would include the main and by‐product revenues. Variable contribution margin is used to indicate the profitability of a process on a fixed‐cost‐free basis. This can be calculated as follows: Eq. 0.14 14 6.5 Economic evaluation of projects Reading: To complete this unit, study chapter 9 in your prescribed textbook, Chemical engineering design. The purpose of investing money in a chemical plant is to earn money; therefore some type of economic evaluation of projects is needed. How would you decide whether a project is economically viable? If a project is making a profit, is that enough to go ahead? Are there other alternatives that would be more profitable? For small projects, and for simple choices between alternative processing schemes and equipment, the decision can usually be made by comparing the capital and operating costs. However, more sophisticated evaluation techniques and economic criteria are needed when decisions have to be made between large, complex projects, particularly when the projects differ widely in scope, time scale and type of project. We will discuss a few techniques used to evaluate the economic viability of projects and techniques for comparing alternatives. 6.6 Cash flow and cash flow diagrams Most economic evaluation techniques require the assessment of the cash flow of a project. A cash flow enables you to create a short‐term forecast that allows you to determine how you are going to get money for the project and pay for your expenses. The cash flows in a manufacturing company can be likened to the material flows in a processing plant. In the same way that we account for material flows into and out of a plant, we can account for the flows of cash into and out of a project. The cash flows into the project would be the revenue. A cash flow diagram, such as that shown in figure 9.1 in the prescribed textbook (or figure 6.8 on this website: https://chemicalprojects.wordpress.com/2014/05/13/economic‐evaluation‐of‐chemical‐engineering‐projects/), shows the forecast cumulative net cash flow over the life of a project. Cash inflows usually arise from financing, operations and investing whereas cash outflows mainly result from expenses. These are based on the best estimates of investment, operating cost, sales volume and selling price that can be made for the project. Towler and Sinnott (2013) describe the key points on the cash flow diagram as follows: A – B: Investment required to design a plant. B – C: Capital required to build the plant, start‐up and initial working capital. C – D: At point C, income is generated with the cash flow turning positive; hence the change in curve. The investment is paid off at point D which is known as the break‐even point. The time taken for point C to D is known as the payback period. D – E: Cumulative cash flow is now positive with the project earning a return on investment. E – F: Slope of the curve begins changing as rate of cash flow declines as a result of increased operating costs and decrease in sales. End of project life is reached at point F. Cash flow is discussed in more detail in the following clip: https://www.youtube.com/watch?v=kega_QOCvxQ&list=PLRW1FgIW06IpkWmpIl_1qrXIPPzZdc7‐V Study example 8.2 in your prescribed textbook. 15 6.7 Depreciation Depreciation is defined as a decrease in value with age. This decrease in value may be due to physical deterioration, technological advances, economic changes, or other factors, which will result in the retirement of the asset. The reduction in value of the asset due to any of these factors is a measure of the depreciation. A machine may depreciate because it is wearing out and is no longer performing its function as well as when it was new. This is called deterioration. Another aspect of depreciation is caused by obsolescence. A machine or equipment is obsolete when the function it performs can be done in another manner or is no longer needed due to technology or market changes. The accounting profession defines depreciation as the allocation of the cost of an asset divided by its useful, or depreciable, life. In determining taxable income it is the accountant's definition of depreciation that is used in the calculations. The economic function can be employed as a way of distributing the original expense for a physical asset over the period during which the asset is in use. From the viewpoint of the design engineer, according to Peters and Timmerhaus (1991), the total cost due to depreciation is the original or new value of an asset minus the value of the same asset at the end of the depreciation period. The original value is usually taken as the total cost of the asset until it is ready to be used for the first time. The value at the end of its life is its salvage value. The engineer cannot wait until the end of the asset's life before the depreciation can be calculated, but it must be used and adjusted throughout the asset's life. The value of the asset at the end of its life, as well as the lifespan of the asset, would not be known at the beginning of the asset’s life, but as the asset's life progresses, the new information can be taken into account and the depreciation adjusted. What is the purpose of depreciation as a cost? If depreciation is deducted as a cost, then the profit earned by the company is evaluated realistically and it provides a basis for the determination of tax. When accountants use depreciation, they must take care to ensure that the government rules on depreciation are adhered to. Service life is that timespan in which the asset is economically feasible. Both physical and functional depreciation must be taken into account when the service life is determined. Salvage value is the amount of money that can be obtained from the sale of the asset after all expenses are deducted. If the asset cannot be sold as a unit, it can be broken up and sold merely as scrap. The profit obtained from selling the asset as scrap is known as the scrap value. Salvage value, scrap value and service life are estimated when the asset is started up and the estimate is made on all the relevant factors known at that specific time. There are various methods for determining depreciation. The methods that will be discussed in this section are straight‐line and declining balance method. The method most used in industry is straight‐line depreciation. 6.7.1 Straight‐line depreciation In this method a constant depreciation charge is made. To obtain the annual depreciation, the total amount to be depreciated is divided by the useful life in years: Eq. 0.15 Where: 16 Watch the following clip for a summary of the straight‐line depreciation method before considering example 6.3: https://www.youtube.com/watch?v=YiVHIFfu5TU Example 6.3 Consider the following: Cost of the asset = R1 000 Service life = 5 years Salvage value = R20 Calculate the straight‐line depreciation. Solution 1 000 20 5 196 Example 6.3: Straight‐line depreciation calculation Book value before Depreciation for the Year depreciation charge year 1 Cost = 1 000 196 2 804 196 3 608 196 4 412 196 5 216 196 Total depreciation = R980 Book value after depreciation charge 804 608 412 216 20 = salvage value This is illustrated in the following figure: 1000 900 R 196 Book Value (R) 800 700 R 196 600 500 R 196 400 R 196 300 200 R 196 100 0 0 1 2 3 4 5 Years, n 17 6.7.2 Declining balance method of depreciation The annual depreciation cost is a fixed percentage of the asset value at the beginning of the particular year. The fixed percentage factor remains constant throughout the service life of the asset, whereas the annual depreciation cost differs each year. At the end of the first year: 1 At the end of the second year: 1 At the end of n years and also the end of the service life: 1 Eq. 0.16 Where: A fixed percentage is often chosen and is usually 50% more than straight‐line depreciation. If the fixed percentage is double that of straight‐line depreciation, then the method is called double declining balance depreciation. Example 6.4 Consider the following: Cost of the asset = R1 000 Service life = 5 years Salvage value = R20 Calculate the double declining balance depreciation. Solution The straight‐line depreciation rate is 1/n = 1/5 = 0.2. So double this rate is 0. 4 0.4 At the end of the first year: 1 1 000 1 0.4 600 At the end of the second year: 1 18 1 000 1 360 0.4 At the end of the third year: 1 1 000 1 0.4 216 At the end of the fourth year: 1 1 000 1 0.4 129.6 At the end of n years and also at the end of the service life: 1 1 1 000 1 0.4 77.6 Note this is higher than the salvage value. In the final year, the asset value should be depreciated to the salvage value Example 6.4: Declining balance depreciation calculation Book value before Year Depreciation for the year depreciation charge 1 Cost = 1 000,00 1 000,00 – 600 = 400 2 600 600 ‐ 360 = 240 3 360 360 ‐ 216 = 144 4 216 216– 129.6 = 86.4 5 129.6 129.6 – 20,00 = 109.6 Total depreciation = R980 Book value after depreciation charge 600 360 216 129.6 20 = salvage value This is illustrated in the following figure: 19 1000 900 Book Value (R) 800 700 600 500 400 300 200 100 0 0 1 2 3 4 5 Years, n 6.8 Rate of return calculations Cash flow figures do not show how well the capital invested is being used; two projects with widely different capital costs may give similar cumulative cash flow figures. Some way of measuring the performance of the capital invested is needed. Rate of return (ROR) is the gain or loss on the investment of a project during a specific period and is a simple index of the performance of the money invested. Though basically a simple concept, the calculation of the ROR is complicated by the fact that the annual profit (net cash flow) will not be constant over the life of the project. The simplest method is to base the ROR on the average income over the life of the project and the original investment: 100 Eq. 0.17 Where: 1 2 3……. In other words, a cumulative cash flow is calculated as follows: if your company begins the period with R60 000 in cash and generates R20 000 in net cash flow, the cumulative cash flow equals R80 000. See the following clip for an alternate explanation: https://www.youtube.com/watch?v=tBkmTV9wyJ4 6.9 Payback period Payback period is the time required after the start of the project to pay off the initial investment from income. This is point C‐D on the cash flow diagram where income is generated with the cash flow turning positive; hence the change in curve. The investment is paid off at point D, which is known as the break‐even point. Payback time is a useful criterion for judging projects that have a short life or when capital is only available for a short time. It is often used to judge small improvement projects on operating plant. Typically a payback time of 3 to 5 years would be expected from such projects. 20 Payback period as a criterion of investment performance does not, by definition, consider the performance of the project after the payback period. Payback does not take the time value of money into account. / Eq. 0.18 This equation can only be used if the cash inflows are equal every year. If the net cash inflows vary, then you need to determine how many years it would take to recover the investment. The decision criterion when payback is used to make accept/reject decisions is as follows: ‐ ‐ If the payback period is less than the maximum acceptable payback period, accept the project. If the payback period is greater than the maximum acceptable payback period, reject the project. 21 Example 6.5 Projects A and B have the following cash flow streams. Which project should be implemented? Example 6.5: Data Initial investment Year 1 Year 2 Year 3 Year 4 Project A ‐12 000 4 000 6 000 6 000 1 000 Project B ‐12 000 2 000 4 000 4 000 8 000 Solution Initial Investment Year 1 Year 2 Year 3 Year 4 Example 6.5: Calculation of payback periods Project A Cash flow Cumulative cash flow Cash flow (12 000) (12 000) (12 000) 4 000 (8 000) 2 000 6 000 (2 000) 4 000 6 000 4 000 4 000 1 000 5 000 8 000 2 2000 6 000 2.33 3 2000 8 000 3.25 Project B Cumulative cash flow (12 000) (10 000) (6 000) (2 000) 6 000 Project A has a payback period of 2.33 years and project B has a payback period of 3.25 years. A firm may decide that any project accepted by the firm must have a payback period of no more than 3 years. Project A would be accepted, but project B would be rejected as its payback period is longer than 3 years. Where a project will earn equal cash flows each year, the payback period can be found by dividing the cost of the investment by the annual cash flow. The payback period method is widely used in practice. It does have the following disadvantages: ‐ ‐ It ignores cash flows after the payback period. It is therefore not a profitability indicator and creates a bias against long‐term projects. For example, project B earns cash flows of R6 000 after the payback period, and project A earns cash flows of R5 000, which were ignored. It ignores the time value of money. When comparing projects, it assumes that the time value of money is zero. Study example 9.2 in the prescribed textbook. Activity 6.5 1. What is the difference between after‐tax profit and after‐tax cash flow? When are these two quantities the same? 2. In general, it is better to depreciate the fixed capital investment as soon as allowable. Give one example of when this statement would not be true. 22 3. What is depreciation? Explain how it affects the economic analysis of a new chemical process. 4. A heat exchanger has been designed and insulation is being considered for the unit. The insulation can be obtained in thicknesses of 1, 2, 3 or 4 inches. The following have been determined for the different insulation thicknesses: Heat exchanger data 1 in 2 in 3 in 4 in BTU/h saved 300 000 350 000 370 000 380 000 Cost for installed R1 200 R1 600 R1 800 R1 870 insulation Annual fixed costs 10% 10% 10% 10% What thickness of insulation should be used? The value of heat is 30 cents/1 000 000 BTU. An annual return of 15% on the fixed capital investment is required for any capital put into this type of investment. The exchanger operates 300 days per year. (Answer: 2” insulation should be recommended.) 5. The fixed capital investment of plant is estimated to be R200 000, and the salvage value of the plant is estimated at 10% of the FCI. Assuming a six‐year project life, estimate the yearly depreciation and book value using a. the straight‐line method b. the declining balance method with f = 2/n (Answer: a) Total depreciation = R180 000; b) Total depreciation = R182 441,70) 6.10 Net present value The money earned in any year can be put to work (reinvested) as soon as it is available and to earn a return. So money earned in the early years of the project is more valuable than that earned later in the project. This time value of money can be allowed for by using a variation of the familiar compound interest formula. The net cash flow in each year of the project is brought to its present value or present worth at the start of the project by discounting it at some chosen interest rate. This rate ‐ often called the discount rate, required rate and cost of capital ‐ refers to the minimum return that must be earned on a project to leave the firm's market value unchanged. / 1 1 Eq. 0.19 Eq. 0.20 Where: The discount rate is chosen to reflect the earning power of money. It would be roughly equivalent to the current interest rate that the money could earn if invested or it could be seen as the company's cost of capital. 23 The total of all the NPV will be less than the total of all the cash inflows and reflects the time value of money and the pattern of earnings over the life of the project. Watch an example of an NPV calculation here: https://www.youtube.com/watch?v=HFFkFMfotT0 Decision criteria for NPV The decision criteria when NPV is used to make accept/reject decisions are as follows: ‐ ‐ If NPV is greater than R0, accept the project. if NPV is less than R0, reject the project. If NPV is greater than zero, the firm will earn a greater return than its cost of capital. This action should enhance the market value of the firm and therefore the wealth of its owners. If more than one project is being evaluated, the one with the higher NPV over R0 should be accepted. Example 6.6: The sales forecast for a new product is estimated as follows: Sales forecast Year Tons 1 2 000 2 2 400 3 3 500 4 4 500 5 1 500 The following economic parameters have been established: Total capital investment (TCI) = R5.2 million Working capital = 15% of TCI Salvage value after 5 years = R1.2 million Fixed costs = R600 000 per annum Variable costs = R320/ton Selling price = R1 320 Tax rate = 48% Using the declining balance depreciation method, calculate the net present value (NPV) at an interest rate of 15%. Solution Declining balance method for depreciation: End of 1st year: Asset value V End of 2nd year: Asset value V 1.2 1 1 V 1 V 1 f 4.42 f 4.42 0.23 4.42 10 1 10 1 0.23 0.23 $ 3.41 10 $ 2.62 10 24 End of 3rd year: Asset value V V 1 f End of 4th year: Asset value V V 1 f End of 5th year: Asset value V V 1 f 4.42 4.42 4.42 10 1 0.23 $2.02 10 10 1 0.23 $ 1.56 10 $ 1.2 10 10 1 0.23 Note that we have only calculated the asset values and NOT the depreciation. The depreciation is the difference between the asset values of two consecutive years, for example D2 = Va1–Va2 Example 6.6 YearBook value before depreciation charge (R)Depreciation for year (R)Book value after depreciation charge (R) 1 4 420 000,00 1 014 553,48 3 405 446,52 2 3 405 446,52 781 675,93 2 623 770,59 3 2 623 770,59 602 252,39 2 021 518,20 4 2 021 518,20 464 013,19 1 557 505,00 5 1 557 505,00 357 505,00 1 200 000,00 TOTAL DEPRECIATION = 3 220 000,00 These are sample calculations for year 1. Calculations for year 1: The tonnage or production rate 2 000 Income / Selling price Variable cost Production rate Selling price given as 1 320 2 000 Cost Production rate 320 2,64 R1 320 ton 10 2 000 640 000 Profit before tax Income Fixed cost 2 640 000 600 000 Variable cost 640 000 Depreciation 1 014 553 R385 447 25 Tax 48% 0.48 R385 447 R185 014 Profit after tax Cash flow R385 447 Profit after tax R200 432 Year R185 014 R200 432 Depreciation 1 014 553 1 214 986 Tons Account for capacity Selling Price (R/ton) 0 1 2 3 4 5 2,000 2,400 3,500 4,500 1,500 Depreciation Fixed Investment/Project life Sales Fixed Expenses Operating Expense (Variable) Tonnes× Selling Price 2,640,000.00 600,000.00 640,000.00 3,168,000.00 600,000.00 768,000.00 4,620,000.00 600,000.00 1,120,000.00 5,940,000.00 600,000.00 1,440,000.00 1,980,000.00 600,000.00 480,000.00 1320 1320 1320 1320 1320 Profit before tax Sales-(OE+D) 1,014,553.48 781,675.93 602,252.39 464,013.19 357,505.00 Tax 48% of Profit 385,446.52 1,018,324.07 2,297,747.61 3,435,986.81 542,495.00 AfterTax 185,014.33 488,795.55 1,102,918.85 1,649,273.67 260,397.60 200,432.19 529,528.52 1,194,828.76 1,786,713.14 282,097.40 Cash Flow Add Depreciation -5,200,000.00 1,214,985.67 1,311,204.45 1,797,081.15 2,250,726.33 639,602.40 Remember to include the total capital investment at year 0. 1 1 1 NPV factor (1+i)n i= 0.15 1.00 1.15 1.32 1.52 1.75 2.01 TOTAL 1 1 1 1 -5,200,000.00 1,056,509.28 991,458.94 1,181,610.03 1,286,860.09 317,995.43 596,612.08 231,045.85 26 Note: The NPV of the salvage value at year 5 is calculated: 1 200 000 2.01 596 612 27 6.11 Internal rate of return (IRR) The IRR is the most used technique for evaluating investment alternatives. It is defined as the discount rate that equates the present value of cash inflows with the initial investment associated with the project. This means that the IRR is the discount rate that causes the NPV to equal R0 (zero). By calculating the net present worth for various interest rates, it is possible to find an interest rate at which the cumulative net present worth at the end of the project is zero. This interest rate is also called the discounted cash flow rate of return (DCFRR) and is a measure of the maximum rate that the project could pay and still break even by the end of the project life. The value of the interest r is found by trial and error calculations. These are normally tedious calculations to do by hand and would be much simpler if done in an Excel spreadsheet. The following website gives you step‐by‐step instructions on how to calculate IRR in an Excel spreadsheet: http://www.investinganswers.com/education/time‐value‐money/calculating‐internal‐rate‐return‐using‐excel‐or‐ financial‐calculator‐2129 1 0 Eq. 0.21 Where: The following clip explains the difference between NPV and IRR: https://www.youtube.com/watch?v=yb8a8QPUYCc&list=PL4xAk5aclnUjEuE_fvbyEts_oBpHYcwLY&index=14 Decision criteria for IRR The decision criteria when IRR is used to make accept/reject decisions are as follows: ‐ ‐ If the IRR is greater than the cost of capital, accept the project. If IRR is less than cost of capital, reject the project. These criteria guarantee that the firm earns at least its required return. This outcome should enhance the market value of the firm and therefore the wealth of its owners. Example 6.7 Calculate the IRR for the scenario presented in example 6.6. Solution To solve for the IRR, we need to use an iterative approach (or trial and error). We choose a value for the interest rate (from the calculation in example 6.6 we note that the NPV is positive at an interest rate of 15%) and determine the NPV as in example 6.6, until we find the interest rate that gives an NPV = 0. In this case, an interest rate = 16.7% gives an NPV = 0 28 NPV factor (1+i)n 1.00 1.17 1.36 1.59 1.85 2.16 -5,200,000.00 1,041,251.80 963,029.59 1,131,153.43 1,214,118.65 295,687.61 554,758.92 0.00 TOTAL See the following clip if you are still struggling to follow the examples: https://www.youtube.com/watch?v=OSDDrZZaV8E Activity 6.6 1. You are trying to decide whether to install an electric geyser, a heat pump or a solar water heater in your home. You have collected the following information: Water heater Investment cost (R) Operating costs (R/annum) Energy consumption (kWh/annum) Electric geyser R7 000 110 6 500 Solar water heater R19 800 300 2 000 Heat pump R15 500 330 2 200 Assume the electricity tariff is R1.4/kWh. a) Determine the payback period for both the heat pump and solar water heater by considering the energy savings of these two technologies when compared to the electric geyser. (Answer: Paybackheat pump = 2.67 yrs and Paybacksolar water heater = 3.24 yrs) b) Determine the ROI for the heat pump and solar water heater. (Answer: ROIheat pump = 37.4% and ROIsolar water heater = 30.9%) 2. A small business with an initial capital investment of R40 000 yields a cash flow of R15 000 during the first year of operation. The yield increases by R1 000 from its second year of operation up to its sixth year of operation. a) Determine the payback period for the project. (Answer: 2.53 yrs) b) Find the NPV of the business assuming an interest rate of 18%. (Answer: Total NPV = R19 547,45) 3. A preliminary design study for a new product established the following economic parameters: Fixed capital investment = R24 000 000 Working capital = R4 000 000 Production at 100% capacity = 2 10 kg/yr Fixed costs = R1 000 000/yr Variable costs = R2,50/kg 29 Selling prices = R9,50/kg The evaluation period is 6 years, the capacity in the first year is 50%, 90% in the second year and 100% each year after. The tax rate is 35%. Use the straight‐line depreciation method and assume salvage value is 0. a) Determine the return on investment (ROI) for the project. (Answer: 27.94) b) Calculate the net present value (NPV) at an interest rate of 15%. c) Calculate the internal rate of return (IRR). 6.12 Summary Having completed this learning unit, you should be familiar with the different types of capital cost estimates and be able to make an equipment module estimate based on data from major process equipment. We briefly considered the use of cost indices to adjust for inflation and data from different locations. We discussed the detailed factorial method in order to make estimates of installed costs. You learnt about total module costs so that you can estimate the capital cost of building a new plant or making modifications to an existing facility. We dealt with methods to evaluate different costs. Towards the end of the learning unit, we discussed profitability analysis to assess the economic viability of a project. Sources consulted Dysert, LR. 2003. Sharpen your cost estimating skills. Cost Engineering, 45(6):22‐30. Peters, MS & Timmerhaus, KD. 1991. Plant design and economics for engineers. 4th edition. New York: McGraw‐Hill. Seider, WD, Seader, JD & Lewin, RL. 2006. Product and process design principles: synthesis, analyses and evaluation. 2nd edition. New Jersey: John Wiley and Sons. Towler, G & Sinnott, RK. 2013. Chemical engineering design: principles, practice and economics of plant and process design. 2nd edition. Oxford: Butterworth‐Heinemann. Turton, R, Bailie, RC, Whiting, WB & Shaeiwitz, JA. 2009. Analysis, synthesis and design of chemical processes. 3rd edition. New Jersey: Prentice Hall. 30 Learning unit 7: Impact of engineering design 7.1 Introduction In this unit, we discuss the importance of occupational health and safety in process design. We introduce safe working conditions as outlined by safety regulations, together with the relevant codes and standards required for a safe working environment. We explain green engineering principles in some depth, which include pollution prevention environmental regulations consequences of manufacturing (life cycle analysis) Finally, you will learn about the concept of ethics in engineering so that you have a sense of the ethical problems you may face as an engineer in the workplace. 7.2 Learning outcomes At the end of this learning unit, you should be able to explain the importance of safety in the design and operation of process industries apply safety legislation with which companies need to comply identify process and material hazards that need to be considered in the process design reduce or eliminate process hazards by considering inherently safe design design a process that will take into account green engineering and pollution prevention carry out a life cycle analysis/assessment in order to evaluate the environmental impact of the design describe strategies that would allow you to make the best choice when faced with an ethical problem 7.3 Health and safety Reading: To complete this unit, study chapter 10 in your prescribed textbook, Chemical engineering design. 7.3.1 Safety legislation A primary objective in the design and operation of chemical plants is to maintain safe working conditions for both the operating personnel and the inhabitants living near the chemical plant. To avoid accidents where extensive damage is caused as well as the loss of lives, companies have introduced extensive safety policies and procedures. The Occupational Health and Safety Act (OHSA) regulates the health and safety of employees. The most significant clause in the OHSA reads as follows: 1 “Each employer shall furnish to each of his employees employment and a place of employment which are free from recognized hazards that are causing or are likely to cause death or serious physical harm to his employees”. This implies that employers should avoid exposing their employees to hazards that are known to them (the employers), whether or not the hazard is identified by the OHSA. This places responsibility for safety solely on the employer. Most engineers are employees, but as they represent the employer, the duty and responsibility lie with them. Process safety management requires employee training, written operating procedures, specific quality in engineering design of components and systems, specific procedures for operating activities, investigation as well as reporting of incidents when they occur and finally an audit of the safety procedures of the company. You can find and download the Health and Safety Act for South Africa from the following link: http://www.labourguide.co.za/health‐and‐safety/848‐health‐and‐safety‐downloads Read up on the history of safety Acts and the events leading to the introduction of safety legislation on Wikipedia: https://en.wikipedia.org/wiki/Factory_Acts 7.3.2 Plant safety Process safety focuses on preventing hazards associated with chemical process facilities or other facilities dealing with hazardous substances. It involves prevention of leaks and spills that may have a serious effect on plants and the environment. Prevention of equipment malfunction, temperature and pressure deviations, corrosion and metal fatigue may lead to hazardous working conditions or incidents and need to be taken into account when considering process safety. If a process is deemed to be inherently safe, then incidents are less likely to occur because an inherently safer design takes into account safety issues in the design as well as operation of chemical and manufacturing processes. The key idea of implementing an inherently safer design is to make a process safer rather than just safe. The safety strategies for a chemical process could be grouped into four categories: inherent, passive, active and procedural: Inherent: An inherent strategy encourages a change in process materials or conditions which will reduce or eliminate the hazard. So to eliminate a hazard, you would either eliminate the hazardous material or change the material or conditions of use. For example, you could substitute water for a flammable solvent if process conditions allow for this. Passive: Passive safety features minimise hazards by using process or design features that would reduce the frequency of an incident occurring without raising an alarm. For example, a containment dike is installed around a hazardous material tank in order to contain any spills. Active: An active safety system would include the use of controls, locks, alarms and safety shut‐off systems. These are installed to prevent incidents or mitigate the consequence of an incident. An example of this could be a high level alarm in a tank that would shut off feed to prevent overflow. 2 Procedural: This would include procedural safety features, standard operating manuals, emergency response procedures and training to be provided to personnel. An example of this would be to educate and provide personnel with confined entry procedures. Don’t think of these strategies as discrete categories with clear boundaries, but rather as a contributing factor towards process safety. The idea of an inherently safe design is to restructure the process in order to avoid any hazard. Turton et al (2009) describe a strategy based on a hierarchy of approaches: 1. Substitution: Avoid producing or using any form of hazardous material. Choose pathways in the process that would eliminate the use or production of hazardous materials. 2. Intensification: Attempts are made to use less of the hazardous material. 3. Attenuation: Implement changes to the process that would reduce the use or production of the hazardous material. This could be lowering the temperature of a path or adding stabilisers. 4. Containment: If the above is not possible, i.e. substitution, attenuation or intensification, make adequate storage arrangements to prevent leaks. 5. Control: Provisions, in the event of a leak of hazardous materials, need to be put in place. This is to ensure that adequate safety procedures are in place to eliminate the effects of the hazardous material. Provision for control measures could be in the form of scrubbing systems, flares, etc. 6. Survival: This is the lowest level of inherent safety design and allows for provision of safety equipment or the survival kits needed for survival of personnel or the equipment if control measures fail. The following video published by the US Chemical Board covers the future of risk reduction by considering an inherently safer design: http://www.csb.gov/videos/inherently‐safer‐the‐future‐of‐ risk‐reduction/ 7.3.3 Material hazards A hazardous material is defined as any item that has the potential to cause harm to humans, animals or the environment. These materials are regulated by different bodies and the OHSA of South Africa (http://www.labourguide.co.za/healthsafety/791‐hazardous‐chemical‐substance‐reg‐1995/file). We will briefly review the hazards of chemicals in this section. 7.3.3.1 Toxicity Toxic substances are not all equally toxic. This means that their relative quantities in the environment play a role in determining their environmental toxicity. Typical methods of emissions abatement rely on effective measurement of concentrations of toxic components in large, dilute streams (such as the flue gas from a boiler in a power station, for example). The designer has to have effective means of measurement available to be able to implement reliable emissions control measures. Effective abatement methods depend on the knowledge of process chemistry and, more importantly, characterisation of the materials used in the process and their associated toxicity. It is important to determine the desired specifications for feed materials in order to minimise waste 3 product generation (including toxic by‐products). This is crucial for successful commercialisation of many chemical processes. Toxic materials could be further classified into two classes: those that cause immediate injury (safety hazards) and those whose effects become apparent over a long exposure time in low concentrations (industrial health and hygiene hazards). Permissible limits and precautions have therefore been set for these two classes to ensure good operating practice. For safety hazards deemed to have short‐term acute effects, toxicity limits have been defined as LD50 (lethal dose 50). This is the lethal dose at which 50% of animals tested with the toxic material have been killed. This dose is represented as the quantity of toxic material (in mg) per body weight (in kg) of the tested animal. Table 10.1 in the prescribed textbook has toxic data for various toxic chemicals. For long‐term chronic effects, a threshold limit value (TLV) has been defined. This takes into account exposure time to the toxic material as well as the inherent toxicity of the material and is expressed in ppm (parts per million) for vapours and gases and mg/m3 for dusts and liquids. 7.3.3.2 Flammability Hazards caused by flammable material could depend on the following factors (Towler & Sinnott 2013): 1. Flash point of material This is the measure of ease of ignition of the material and is the lowest temperature at which the material will ignite from an open flame. Data for flashpoints can be found in handbooks such as Perry’s Chemical engineers handbook. 2. Auto‐ignition temperature of the material This is the temperature at which spontaneous combustion could occur without any external sources of ignition. 3. Flammability limits This is defined as the limit of a material at the highest and lowest concentrations at which a flame will propagate through the mixture. See table 10.2 in the prescribed textbook for flammability ranges for a number of materials. 4. The energy released in combustion This is the energy released when a chemical burns in the presence of oxygen to produce carbon dioxide and possibly water. 7.3.3.3 Safety data sheets Section 9 of the Act requires that every person who manufactures, imports, sells or supplies any hazardous chemical substance for use in any way is responsible for the safe use of the product. That person must provide a safety data sheet (SDS) (previously called a material safety data sheet, MSDS) that has the information applicable to the particular substance as outlined here: product and company identification composition/information on ingredients hazards identification 4 first‐aid measures fire‐fighting measures accidental release measures handling and storage exposure control/personal protection physical and chemical properties stability and reactivity toxicological information ecological information disposal considerations transport information regulatory information other information The SDS for every chemical should be made available to both the employees of the industry as well as the customers to ensure safe handling and transportation of the product. The SDSs are normally created by the manufacturers of the substance. Have a look at SDSs for chemicals found in common household detergents, as examples, on the following websites: Ammonia commonly found in Handy Andy: http://www.afrox.co.za/internet.global.corp.zaf/en/images/Ammonia%20%28Rev%203%29266_275 91.pdf?v=2.0 Dodecylbenzene sodium sulfonate commonly found in laundry detergents: https://www.spectrumchemical.com/MSDS/D3581.pdf Linear alkylbenzene sulfonate commonly found in Sunlight dishwashing liquid: http://www.labchem.com/tools/msds/msds/75441.pdf 7.3.4 Process hazards Process hazards are defined as hazards associated with an industrial process. These types of hazards have the potential to cause large‐scale damage, resulting in downtime and loss of life. To minimise process hazards, it is important that equipment be regularly maintained and inspected. Once a hazard has been identified, the level of risk is determined and the appropriate safeguards should be put in place to provide a basis of safety. These are discussed in detail in section 10.3 of the prescribed textbook and we have summarised them briefly here: 1. Pressure: Exceeding operating pressure has been identified as the most serious hazard in the process industry. This results in failure of the pressure vessel, which could precipitate events leading to a major disaster. Over‐pressure is normally relieved by the installation of a pressure relief device. The following CSB video Blocked in highlights the importance of pressure relief devices and their proper installation: http://www.csb.gov/videos/blocked‐in/ 5 2. 3. 4. 5. 6. 7. Temperature: Excessive temperature may cause structural damage or failure and is common in reactors as well as heaters. To eliminate the process exceeding the maximum operating pressure, temperature alarms are installed and often emergency cooling systems are provided. Noise: High noise levels do not promote a good working environment and damage to hearing may occur. It is recommended that equipment producing high noise levels be installed away from the control room as well as local communities. Loss of containment: This is defined as an unplanned or uncontrolled release of material from primary containment, including non‐toxic and non‐flammable materials. It may be a result of various errors such as pressure relief failure or poor maintenance procedures. This CSB video highlights a crucial process hazard due to loss of containment of a flammable material: http://www.csb.gov/caribbean‐petroleum‐refining‐tank‐explosion‐ and‐fire/ Fires and ignition sources: Various ignition sources such as electrical equipment and flames from process operations such as furnaces, flares and incinerators could act as a source for ignition or cause a rapid fire. To control the fire, flame traps and fire protection may be implemented. Explosions: Explosions could occur as a result of over‐pressurising the vessel or even ignition of a flammable mixture. Flammable materials are normally designed in such a way that the possibility of an explosion occurring is limited and eliminated as far as possible. Human error: The possibility of human error will always exist and good training procedures are encouraged in order to avoid human error. 7.3.5 Analysis of product and process safety The analysis of health, safety and the environment is an important aspect of the design process and must be carried out at every stage of the project. At each stage of the project, a hazard analysis is required to break down each job into its components, evaluate each stage and identify hazards associated with the stage. The hazard then needs to be eliminated or appropriate measures need to be taken, such as recommending appropriate personal protective equipment or specifying safe worker procedures. In this way, hazards are determined and proper work procedures could be established. In the early stages of the project, information about the chemistry of the process is known, as well as the major pieces of equipment that will be utilised. At this stage an SDS review can be carried out to gauge the hazards associated with the chemistry of the process. Pollution prevention analysis can also be recommended if the outputs in terms of effluent are known. At the preliminary design stage, more information about the process is available, including the mass and energy balance, thus allowing for detailed hazard and operability (HAZOP) studies to be carried out. Safety checklists are also completed during this stage, as are defining operating and emergency procedures. 7.3.5.1 Safety checklists 6 Safety checklists provide methods for analysing equipment, workspaces and tasks. They are a useful guide for a less experienced engineer and a good tool in providing clear documentation and acting as a guide to safety procedures. Checklists are intended to question the engineer and promote thought around the safe use of equipment or working conditions. Safety checklists are discussed in more detail in the prescribed textbook, chapter 10.4.1. A sample of part of a reactor vessel checklist is provided in table 7.1. Table 7.1: Sample of a safety checklist for a reactor vessel Inspection Checklist for Process Unit Reactor Vessel Report No: Date Prepared: Equipment Tag: Location/area: Date Inspected: Reason for Inspection: Inspector: Line Manager: Equipment Data DESCRIPTION SHELL TUBE DESCRIPTION Material SHELL TUBE Design P (Psig) Thickness (in) A = Acceptable, U = Unacceptable, NA = Not Applicable, NI = Not Inspected No. Item 1.0 Elbow inlet 1.1 Check elbow inlet for excessive scaling, mechanical damage 1.2 Check flanges and gasket faces 2.0 Top Head and Manway Nozzles 2.1 Internal erosion, cracking, describe appearance and depth A U NA NI Comments 7 2.2 Scale presence, describe 2.3 Welding condition? 2.4 Condition of manway gasket face 2.5 Check condition of insulation 2.6 Inspect internal deflection plates 7.3.5.2 Hazard and operability (HAZOP) studies A HAZOP study is a structured and systematic examination of an existing process or operation to identify and evaluate problems that may represent risks to both personnel and equipment. The study is carried out to review the design and identify engineering issues that may otherwise not have been found. HAZOP studies should not be confused with a hazard analysis. Although similar in nature, a HAZOP study is much more detailed and rigorous. The basic principles when undertaking a HAZOP are to carry out the inspection vessel by vessel and line by line with “guide words” used to identify deviations from the operating procedures or exposure to a hazardous situation. Table 10.10 in the prescribed textbook gives a list of recommended guide words to be used as well as scenarios where these may be used. The following video clip discusses HAZOP studies in some detail together with industry‐related examples: https://www.youtube.com/watch?v=AYtBUkjbVWc Activity 7.1 1. List the hierarchical steps of inherently safe design. 2. What is the purpose of the OHS Act? 3. Consult the OHS Act as provided in the link: http://www.labourguide.co.za/health‐and‐ safety/848‐health‐and‐safety‐downloads. List two general duties of the employers to the employees. 4. Consult the SDS for ammonia as provided in the text. a) List the main hazards as identified in the SDS. b) If ammonia gets into a person’s eye, what is the recommended first aid treatment as described in the SDS? c) List the environmental precautions for ammonia. What measures would you suggest to avoid this? 5. List the process hazards to consider when undertaking a process design. 6. Consult the hazardous chemical substance (HCS) regulations available at http://www.labourguide.co.za/healthsafety/791‐hazardous‐chemical‐substance‐reg‐ 1995/file 8 How would you recommend the disposal of HCS waste? 7. Noise‐induced hearing loss regulations are available on the website: http://www.labourguide.co.za/healthsafety/793‐noise‐induced‐hearing‐loss‐reg‐2003/file a) You need to wear ear defenders but an ear pad is missing from one of the shells. What should you do? b) List the checks you need to carry out when using hearing protective equipment. 8. On 22 May 2017, a blast took place at Sasol’s Natref refinery as reported by Business Live: https://www.businesslive.co.za/bd/companies/energy/2017‐05‐23‐blast‐shuts‐part‐of‐ sasols‐natref‐refinery/. It has been reported that the explosion occurred near the plant’s hydrogen compressors. Hydrogen handling and safety measures are summarised by the Linde article http://www.linde‐ gas.nl/internet.lg.lg.ndl/en/images/Handling%20of%20Hydrogen162_72941.pdf. Read through the article and suggest safety measures that could be put into place. 7.4 Environmental issues Reading: To complete this unit, study chapter 11, section 5 in your prescribed textbook, Chemical engineering design. In recent years, a transformation of environmental protection from a secondary to a primary issue has resulted in tighter environmental regulations. As a result, a noticeable decrease in air and water pollution, as well as remediation of waste dump sites have occurred, with the emphasis on maintaining a cleaner environment. Implementation of regulations has seen a marked improvement in air quality (particularly in urban areas), reduction in water pollution and reduction in toxic waste dumping. Large investments in environmental emission abatement technologies had to be made by the industry in order to remain in the market. These changes have brought about fundamental questioning of process designs, their sustainability and efficiency. While it is true that the initial improvements in emissions abatement were largely made at the expense of the customer, the new environmentally conscious approach to industrial design has brought about efficiency improvements that were previously unexploited. For example, early environmental abatement techniques involved removing toxic components from industrial waste (effluent). Soon it became apparent that, through novel techniques and applications, it is more efficient to reduce the total amount of waste (waste minimisation) and reduce/eliminate processing steps which would result in formation of toxic components and waste from the onset. This requires evaluation of processes in order to protect the environment as well as minimise resource and energy consumption to allow for sustainability. To achieve sustainability, waste management cannot be solved solely with end‐of‐pipe solutions, but instead an integrated approach is required. A waste management hierarchy dictates an order of preference allowing for reduction of waste while at the same time extracting the maximum practical benefits from a product. This could be classified as the following: Avoidance: reduce amount of waste at all levels 9 Resource recovery: re‐use, recycling, reprocessing, energy recovery and efficient use of resources Disposal: dispose in an environmentally responsible way This hierarchy is often presented as a pyramid capturing the progression of material through successive stages of waste management. Figure 7.1 depicts the waste management hierarchy diagrammatically. Despite the notion that chemical processes can be designed to produce no effluents, this is most commonly not the case. A vast number of processes employed in generating some of the most important chemicals also generate a variety of liquid, gaseous and solid waste. Figure 6.1: Waste management hierarchy (Source: https://en.wikipedia.org/wiki/File:Waste_hierarchy.svg) Environmental legislation in developed countries and a considerable portion of the developing countries limits the amounts of effluent that can be emitted to the environment. In most cases, the limits are imposed on continuous emissions (i.e. from stacks, or liquid waste to river) by specifying maximum permissible concentrations of toxic components in such streams. Additionally, a provision is made for once‐off emissions to cater for process upsets, emergencies, etc. All these considerations are used as inputs into the design process. Designers have to determine the optimum process scheme that would maximise production of the desired product (and hence profit) for a given amount of effluent generated (determined by legislation and environmental permits). Sustainability of the process dictates that the designer has to understand and anticipate future developments in environmental legislation, which ties together with the understanding of the chemistry of the process and raw materials used in processing. Environmental considerations are therefore most commonly translated into financial considerations. 10 7.4.1 Environmental regulations In terms of design methodology, some of the environmental regulations can be treated as constraints of the process under consideration. When developing a mathematical process model (process simulation), the designer can check if these limits have been satisfied for selected operating conditions. Design variables can be adjusted to obtain maximum product conversion while maintaining conditions such as to minimise production of unwanted by‐products (i.e. waste). The designer often has to consider other factors which are difficult to quantify. One of these factors is public expectation or perception of pollution, which needs to be considered by the designer in addition to the legislative provisions. For example, a plant that releases small quantities of a specific chemical (which are deemed safe by the regulator), which, despite the low levels, results in a noticeable odour, would likely result in public backlash against the producer/manufacturer from any communities located downwind from the plant who would associate the odour with the particular operation. Governments typically regulate location of industries far away from residential areas, which can significantly increase construction costs. To prevent further restrictions in regulations, which increase costs and complexity of new and existing plants, the plant owners (industry) typically want to ensure that the public has confidence in their adherence to environmental legislation and excellent safety records are maintained. You can download environmental regulations for South Africa (air and water Acts) from the following websites: Clean Air Act: https://cer.org.za/wp‐content/uploads/2013/12/Framework‐for‐Air‐Quality‐ Management_new.pdf Clean Water Act: http://www.dwaf.gov.za/Documents/Legislature/nw_act/NWA.htm National Environmental Management Act (NEMA Act): https://www.acts.co.za/national‐ environmental‐management/index.html In the remainder of the section, we will discuss several specific environmental issues which we have singled out due to their relation to the design and operation of chemical processes. 7.4.2 Environmental issues in process design 7.4.2.1 Burning fossil fuels for power generation and transportation The use of fossil fuels in power generation and transportation is one of the primary sources of compounds contributing to the enhanced greenhouse effect and climate change. The presence of combustion products is largely a function of the impurities present in the fuel. Fuels can be gaseous (natural gas), liquid (petrol, diesel, kerosene, etc.) or solid (coal). Some of the notable impurities are sulphur, phenolics, volatile organic compounds and heavy metals (particularly in coal), while all combustion processes produce to varying degrees SOx (sulphurous oxides), NOx (nitrous oxides) and organic gases (CO and CO2). SOx compounds typically contribute to the acid rain phenomena through 11 the formation of sulphuric acid (H2SO4) when combined with atmospheric moisture. Similarly, NOx compounds react with other organic gases to deplete the ozone (O3) layer. South Africa has played an integral part by signing global agreements regarding climate change. The Paris Agreement was signed by the Minister of Environmental Affairs in 2015, which will guide international efforts to limit greenhouse gas emissions and to meet all the associated challenges posed by climate change. The primary objective of the agreement is to limit the global temperature increase to well below 2 ˚C, while pursuing efforts to limit the increase to 1.5 ˚C. The agreement also aims to implement the following to adapt to climate change: decarbonised electricity by 2050 carbon capture and storage electric and hybrid electric vehicles introduction of a carbon tax If you want to read up about the Paris Agreement, see the following links: http://www.sanews.gov.za/south‐africa/sa‐signs‐paris‐agreement‐climate‐change http://unfccc.int/paris_agreement/items/9485.php Pollution control methods have advanced together with the discovery of the extent of harm that the specific compounds cause. For example removing sulphur from fuels, using catalytic converters to react flue gases from combustion processes and adjusting process parameters in the combustion process to yield less NOx gases are just some of the techniques employed to reduce the harm from the use of fossil fuels. At present, it is more economically sound to treat the effluent streams (i.e. flue gases from the combustion process) than to treat the fuel itself. This, of course, varies with the specific type of fuel and application, but is certainly the case in large energy‐intensive processes (e.g. burning coal to generate power). Activity 7.2 Levels of carbon dioxide emissions for South Africa are available on the Department of Environmental Affairs’ website: http://soer.deat.gov.za/570.html. From the graph provided, you can see that we have been steadily increasing our CO2 levels with no progress being made to decrease them. South Africa is the largest emitter of greenhouse gases on the continent and has pledged to reduce the emissions by 42% by 2025 (Mail & Guardian 2015). Read how greenhouse gas reduction potentials could be achieved in the article “Behaviour change needed to reduce SA's greenhouse gas emissions” by Engineering News: http://www.engineeringnews.co.za/article/behaviour‐change‐needed‐to‐reduce‐sas‐ greenhouse‐gas‐emissions‐2009‐01‐23. Summarise emission levels based on the articles you have read and suggest some changes we as citizens of South Africa could implement in achieving a reduction in greenhouse gas emissions. 12 7.4.2.2 Handling toxic wastes Chemical and nuclear industries (such as power generation, paper and pulp, ore‐refining activities, etc.) are responsible for the generation of large quantities of toxic liquid and solid waste. The vast majority of these waste materials are buried underground (i.e. nuclear) or above ground, depending on the nature and toxicity of the material. A small portion of the waste is incinerated. We generally find that toxic waste will eventually lead to contamination of underground water streams (regardless of the type of storage ‐ underground or above ground), and may also lead to atmospheric contamination (i.e. through dust). There are numerous examples locally and internationally where toxic waste dumps have compromised the health of communities adjacent to or downwind of waste burial sites. Typical water contaminants include heavy metals and organics. Some industrial processes, such as ore processing, lead to the generation of vast quantities of liquid/solid toxic wastes, typically stored in large dams above ground (e.g. the tailings dams left over from gold mining activities, scattered around the eastern and western parts of Gauteng, contain a number of different types of heavy metals and low activity radionuclides). Numerous incidents of water pollution on a massive scale have been recorded globally as a result of tailings dams’ failures (see http://www.wise‐uranium.org/mdaf.html) Activity 7.3 Acid mine drainage is the flow of polluted and contaminated water into the surface and underground water surfaces. This occurs primarily around South Africa’s old mining areas and may contain high levels of salts, sulphate, iron and aluminium, toxic heavy metals such as cadmium and cobalt, and radioactive elements. The impact of acid mine drainage is summarised in the following article: http://www.sajs.co.za/sites/default/files/publications/pdf/712‐5387‐3‐PB.pdf Read the article published in the Mail and Guardian https://mg.co.za/article/2010‐12‐10‐ the‐acid‐mine‐drainage‐solution‐bandwagon and suggest ways that the impact of acid mine drainage could be reduced. 7.4.2.3 Bioaccumulated chemicals Bioaccumulation refers to the accumulation of toxic substances, such as pesticides, heavy metals (such as mercury or cadmium) and radioactive materials in biological organisms. These toxic substances may cause a number of defects through the lifespan of the organism, and can affect a number of species in the particular habitat or food chain. The most common example of bioaccumulation is the very toxic pesticide DDT (1,1‐bis(4‐chlorophenyl)‐2,2,2‐trichloroethane ‐ C14H9Cl5) as well as the polychlorinated biphenyls (PCBs). The former was used extensively in the 1950s to protect crops, forests and even malaria control by insect eradication. After it was discovered that entire ecosystems (from birds and animals to humans) were affected by DDT's toxic effects, it was banned in 1972 (USA). Its effects are, however, still present to this day due to its low rate of degradation and high rate of bioaccumulation in the areas where it was used. 13 PCBs were used extensively in the 1960s in a number of applications, but predominantly as a coolant for electrical transformer stations. They were banned in 1978 (USA) when it was discovered that they are a very potent carcinogen. 7.4.2.4 Toxic metals and minerals Major findings around the toxicity of heavy metals, such as mercury, cadmium and lead, and minerals, such as asbestos, on humans and animals were made towards the end of the 1960s. Mercury, which was once used extensively in gold ore processing and the production of chlorine and caustic soda, was found to cause damage to the brain, kidneys and lungs. Its use is now largely limited to specialty applications, and its emissions strictly controlled and regulated. Lead was once used as an additive to paints as well as in fuel as an octane booster (tetraethyl‐lead). Since the effects of lead poisoning have been confirmed (brain damage, disfigurement, paralysis), its use has been minimised (hence you will observe the prevalence of unleaded petrol and lead‐free paints). Activity 7.4 Impact of over‐exposure to asbestos is discussed in this article: https://www.asbestos.com/mesothelioma/south‐africa/. You work in an environment that allows for occupational exposure limit of 0,2 regulated asbestos fibres per millilitre of air averaged over any continuous period of 4 hours. What precautions and control of exposure will you ensure in your work environment? List the necessary precautions your employer needs to take to create a safe working environment. How would you dispose of asbestos‐ containing products? You can download asbestos regulations from the following website: http://www.labourguide.co.za/healthsafety/246‐asbestos‐regulations‐2001pdf/file and they will assist you in making a reasonable conclusion. 7.4.3 Green engineering With the new knowledge of the effects of toxic materials in the environment, and the associated legislative control over environmental emissions, a need for designing environmentally sound chemical processes has arisen. Chemical engineers throughout the years have developed a number of different methods/techniques to reduce (minimise) the extent of waste generation, avoid the use of toxic compounds and decrease energy use. The latter is an example of the new type of thinking in chemical process design ‐ sustainability. Sustainability implies reduction of waste generation throughout the product life cycle and encompasses both the upstream and downstream processes (i.e. processes generating inputs for a particular (primary) process are upstream processes, whereas further processing or use of products from the primary process are downstream processes). Therefore, it is natural that energy efficiency would be optimised to both reduce production costs, as well as reduce greenhouse gas emissions from upstream, power generation processes. 14 The new paradigm for the environment encourages green chemistry and engineering where the need to eliminate and reduce hazards to the environment is encouraged rather than the traditional environmental approach where end‐of‐pipe solutions are considered. Green engineering means design for reduction of emissions design to eliminate particularly hazardous chemicals minimise the use of natural resources minimise energy consumption In terms of green chemistry, the major issues are as follows: Find alternative feedstocks that are less hazardous and improve environmental performance. Implement the use of green solvents that are less toxic to humans and the environment. Develop new synthesis pathways to avoid the formation of hazardous intermediates or by‐ products. We discuss some of the techniques in further detail. 7.4.3.1 Reaction pathways to reduce by‐product toxicity Production of primary products in a chemical process is very often followed by generation of significant quantities of unwanted, toxic by‐products. Different reaction pathways can result in generating varying degrees of unwanted by‐products, and the aim of the design process is to determine an optimal reaction pathway that would minimise the generation of these products. Firstly, reducing the amount of unwanted by‐products reduces the amount and size of the equipment which is required for both the primary process and the process of handling the unwanted by‐products. This has a direct impact on the economics of the design. Secondly, the methods of toxic emissions abatement (i.e. removal of toxic components from the generated waste, or adequate disposal of waste which cannot be treated) are capital intensive. 7.4.3.2 Avoiding non‐routine events When designing a chemical process, designers have to consider not only the steady state conditions, but also transient states. Examples of transient states are the plant start‐up, shutdown and emergencies. Some of these transients result in waste, which may be in higher concentrations than experienced during normal operation. The duty of the designer is to analyse all aspects of the process design and firstly, identify the effects of transient states on the process, and secondly, introduce additional engineering steps to minimise their effect on the stability and safety of the process. 7.4.3.3 Reducing and reusing wastes Generally, formation of by‐products in chemical processes to some extent is unavoidable. Under such circumstances, the designer can, if the chemistry allows (see different reaction pathways), choose to design a process which would generate a reusable waste product, with minimal or no additional processing. Examples include generation of fertilisers, organic and inorganic acids, etc. 15 7.4.4 Pollution prevention A key issue when trying to prevent pollution during a process design is to minimise generation of waste products in the reactor, design separation systems for maximum recovery and minimum energy usage and minimise effluent streams containing waste. Very often leaks during the transportation process are neglected by the engineer and the impact of this is ignored. Although pollution prevention is specific to the industry and evaluation is industry specific, the following factors are likely to be applied to all potential and actual pollution‐generating activities, including those found in the energy, agriculture, consumer and industrial sectors: Raw materials: These form the largest operating cost and their efficient use is encouraged to ensure economical process operation. If they are not separated and recycled, subsequent reaction could occur, leading to unwanted emission or combustion. When taking into account raw materials, a balance between green engineering and economics is needed. Heat integration: As with raw materials, a balance in green engineering and economics when considering heat integration is needed. Heat integration is implemented to allow for minimum utility usage. Green chemistry: As mentioned previously, chemical reaction should be selected to minimise the formation of toxic products or unwanted by‐products as far as possible. Methods for maximising selectivity of the desired product should be explored and encouraged. Separation processes: As no separation process is perfect, this remains a key issue in pollution prevention. Trace amounts of toxic waste are likely to exist in the pure stream. As far as possible, try to minimise the trace amounts being discharged as effluent. Minimising leaks: These should take into account leak prevention during transportation as well. This area has been neglected in the past. Recycle analysis: Ensure that all recycle opportunities in the process have been exploited before finalising the preliminary design. Pollution prevention reduces both financial and environmental costs. These include reduction of waste management and clean‐up costs as well as health and safety issues that may arise. Furthermore, it protects the environment by conserving and protecting natural resources while strengthening economic feasibility through efficient production in industry and minimising waste handling both in industry as well as our communities. 7.4.5 Life cycle analysis Designers must also consider the entire life cycle of the primary product, for example the secondary uses for the product once its use for the original, primary purpose has been depleted. Product/process design has to include aspects which would enable reusing and/or recycling the product once its useful life has ended. This is known as a life cycle analysis and considers the environmental consequences of a product across its entire life cycle. The life cycle can be divided into the following steps according to Turton et al (2009): Define boundaries of the analysis, i.e. a broad scope of analysis is preferred in order to obtain a better picture of the life cycle of a product. 16 Inventory analysis: There must be a material and energy balance for the product as well as consideration of water, air and waste outputs. Impact analysis: Consider the environmental impact of inputs and outputs. Improvement analysis: Identify opportunities for improving the environmental impact. Examples are modifying the process or making recommendations that may improve the environmental consequences. The life cycle itself is reliant on the following: raw material procurement manufacturing materials product manufacture product use and reuse product and equipment disposal An example of this could be the manufacture of plastics. The actual plastic is manufactured from a variety of low and high density polyethylene, which in turn is made from pure ethylene. The source of ethylene is as a result of an oil refinery by‐product, so the refinery forms part of the analysis too. If we want to broaden the life cycle boundary further, we could take into account the fossil fuel raw material used in the oil refinery as well as the equipment used in the manufacture of the plastic. We also need to define what would happen to the plastic product after use: is it recycled or does it end up in a landfill? As you can see, the life cycle analysis could become very complex, particularly if we include all aspects of the product from raw material to disposal. 7.5 Ethics “Ethics is a (rational) study of moral dilemmas in (human) action. Morals are [briefly] defined as codes or guides of conduct (implicit or explicit) that are based on personal long‐lasting beliefs and values or those of society. A personal act can be considered moral, immoral or amoral from the point of view of ethical studies” (https://ccit333.wikispaces.com/Ethics+and+Design ) Engineers are required to make decisions about issues such as safety and sustainability in design processes. When undertaking the design phase of the project, the engineer needs to question the effect of the design on the environment as well as the sustainability of the design. These effects are ethically relevant because protecting the environment and sustainability are moral issues. Engineering ethics is concerned with the personal conduct of engineers as they uphold and advance the integrity, honour and dignity of engineering while practising their profession. Engineering ethics outlines obligations to ourselves, the employer or client, colleagues and co‐workers, the public and the environment. The specific obligations are set out in the codes of ethics outlined by various engineering societies. The following is an example from the Engineering Council of South Africa (ECSA): Competency Registered persons: 17 a) Must discharge their duties to their employers, clients, associates and the public with due care, skill and diligence b) May only undertake work which their education, training and experience have rendered them competent to perform and is within the category of their registration Integrity Registered persons: a) Must discharge their duties to their employers, clients, associates and the public with integrity, fidelity and honesty b) Must not undertake work under conditions or terms that would compromise their ability to carry out their responsibilities in accordance with the norms of the profession c) Must not engage in any act of dishonesty, corruption or bribery Environment Registered persons must at all times: a) Have due regard, and in their work avoid or minimise, adverse impact on the environment, and b) Strive to ensure that in meeting present development needs, the ability of future generations to meet their needs is not compromised You can download the complete code of conduct https://www.ecsa.co.za/regulation/RegulationDocs/Code_of_Conduct.pdf for ECSA from: Find out more about the need for engineering ethics discussed by Lorraine Doherty in her article published by Creamer Media: http://www.engineeringnews.co.za/article/do‐engineers‐need‐ethics‐ 2014‐08‐01 Activity 7.5 1. The following website gives a case study of withholding relevant information about hazardous waste and its disposal: http://www.onlineethics.org/Resources/32828/HazardousBER.aspx Read through the article and discuss the ethical obligation of the engineer in relation to the code of conduct as provided by ECSA: https://www.ecsa.co.za/regulation/RegulationDocs/Code_of_Conduct.pdf 2. The following case study has been made public by ECSA regarding the collapse of a structure arising from faulty welding of steelwork: https://www.ecsa.co.za/RegisterDocuments/CaseStudy/CaseStudy_2012‐4.pdf Read through the article and comment on the lessons that design engineers could learn. For further enrichment, the site http://www.onlineethics.org/Resources.aspx?resource‐ type=29_772&topic=26_690 has additional case studies involving ethical responsibilities of engineers and technical teams, which may be of interest to you. The article by the American Institute of Chemical Engineers highlights the responsibility of engineers and the ethical responsibilities they face: https://www.aiche.org/sites/default/files/cep/20150221.pdf 18 7.6 Summary In this module, we introduced you to key concepts to ensure a safe and environmentally friendly design. You learnt to apply these concepts and laws in ensuring a safe and ethically viable design process, but many of these laws and regulations may be applicable in your home and community. We discussed the need to identify process and material hazards and the elimination of hazards by considering an inherently safe design. We highlighted the importance of designing a process that takes into account green engineering and pollution prevention together with the use of a life cycle assessment in order to evaluate the environmental impact of the design. As you pursue your career in chemical engineering, you will find that you will be able to apply the network of concepts and regulations that you learnt in this module to a wide range of science and engineering problems in the industry. Sources consulted Mail & Guardian. 2015. SA climate plans inadequate. https://mg.co.za/article/2015‐10‐05‐ sa‐climate‐plans‐inadequate [Accessed 19 September 2017]. Towler, G & Sinnott, RK. 2013. Chemical engineering design: principles, practice and economics of plant and process design. 2nd edition. Oxford: Butterworth‐Heinemann. Turton, R, Bailie, RC, Whiting, WB & Shaeiwitz, JA. 2009. Analysis, synthesis and design of chemical processes. 3rd edition. New Jersey: Prentice Hall. Waste management hierarchy. (n.d.). Retrieved from https://en.wikipedia.org/wiki/File:Waste_hierarchy.svg [Accessed 19 September 2017]. 19