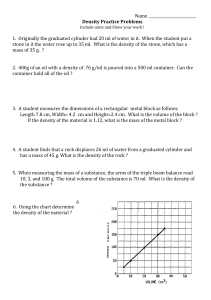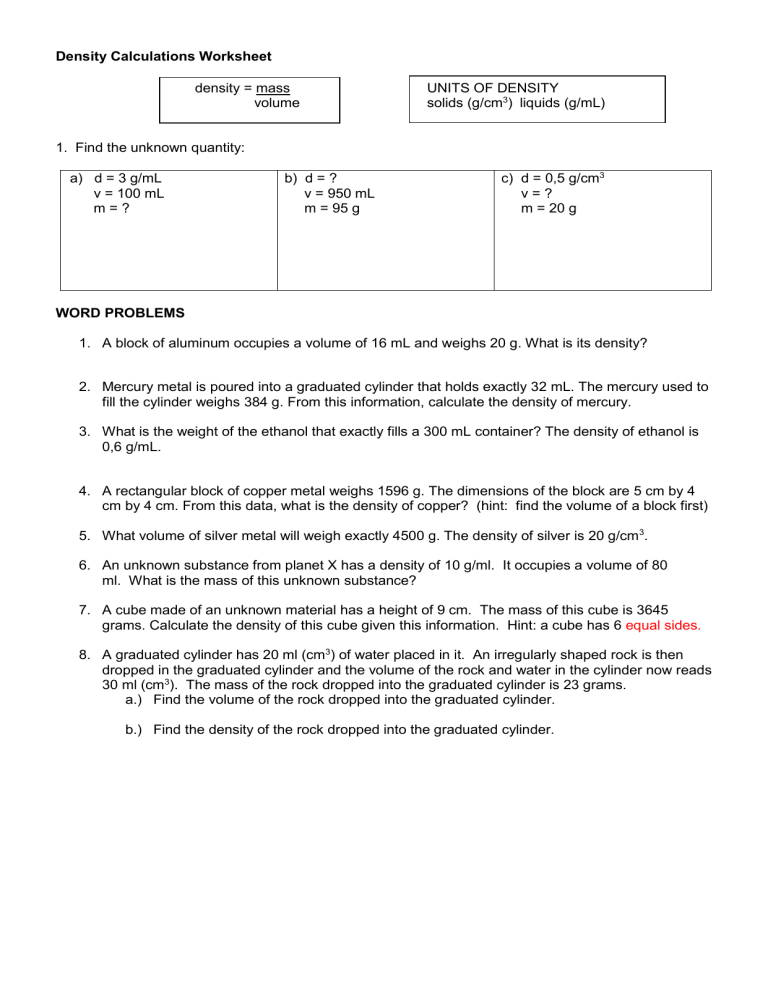
Density Calculations Worksheet density = mass volume UNITS OF DENSITY solids (g/cm3) liquids (g/mL) 1. Find the unknown quantity: a) d = 3 g/mL v = 100 mL m=? b) d = ? v = 950 mL m = 95 g c) d = 0,5 g/cm3 v=? m = 20 g WORD PROBLEMS 1. A block of aluminum occupies a volume of 16 mL and weighs 20 g. What is its density? 2. Mercury metal is poured into a graduated cylinder that holds exactly 32 mL. The mercury used to fill the cylinder weighs 384 g. From this information, calculate the density of mercury. 3. What is the weight of the ethanol that exactly fills a 300 mL container? The density of ethanol is 0,6 g/mL. 4. A rectangular block of copper metal weighs 1596 g. The dimensions of the block are 5 cm by 4 cm by 4 cm. From this data, what is the density of copper? (hint: find the volume of a block first) 5. What volume of silver metal will weigh exactly 4500 g. The density of silver is 20 g/cm 3. 6. An unknown substance from planet X has a density of 10 g/ml. It occupies a volume of 80 ml. What is the mass of this unknown substance? 7. A cube made of an unknown material has a height of 9 cm. The mass of this cube is 3645 grams. Calculate the density of this cube given this information. Hint: a cube has 6 equal sides. 8. A graduated cylinder has 20 ml (cm3) of water placed in it. An irregularly shaped rock is then dropped in the graduated cylinder and the volume of the rock and water in the cylinder now reads 30 ml (cm3). The mass of the rock dropped into the graduated cylinder is 23 grams. a.) Find the volume of the rock dropped into the graduated cylinder. b.) Find the density of the rock dropped into the graduated cylinder. Use the graph below to answer the following questions: C 25 Mass (g) 20 15 10 B A 5 0 5 10 15 20 Volume (cm3) 1. What is the density of object A? Does it sink or float in water? 2. What is the density of object B? Does it sink or float in water? 3. What is the density of object C? Does it sink or float in water? 25 30