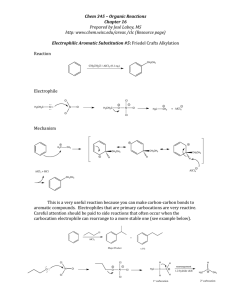
O || NH – C – CH3 CH3 13. Rank the following reactions A, B and C in order of increasing rate, Me Me Me 1. A C B SO3/H2SO4 (A) Identify the position where electrophilic aromatic substitution (EAS) is most favourable. (a) A (b) B (c) C (d) A and C NO2 CH3 Cl SO3H SO3/H2SO4 SO3H (d) (c) SO3H NO2 (C) (b) SO3/H2SO4 (B) NO2 2. (a) Me Correct order of rate of EAS (electrophilic aromatic substitution) is : (a) c > b > a > d (b) c > d > a > b (c) a > b > c > d (d) c > d > b > a (a) B > A > C 14. (b) B > C > A (c) A > B > C (d) A > C > B Rank in order of increasing rate of reaction towards EAS FeBr 3 . CO 1.SOCl 2 AlCl 3 Zn— Hg, HCl, heat O ¾1. ¾¾¾ ® A ¾ ¾¾¾¾¾¾® B ¾ ¾¾¾® C. + 6. with bromine in the presence of 2. AlCl 3 3. H 3O + 2. H 3O + CO (A) The end product (C) is : O (a) (B) (C) (a) B < A < C (c) A < B < C (b) O (b) B < C < A (d) A < C < B O O O C — C6H5 C (c) 15. Identify the position where E.A.S. can take place. (a) 1 (b) 2 (c) 3 (d) C — C6H5 COOH O (d) 4 1 7. How many benzylic hydrogens are present in the hydrocarbon shown below ? 2 O 3 17. (a) 3 (b) 4 (c) 5 8. The major product formed in the reaction is : (d) 6 4 (a) 1 (b) 2 (c) 3 (d) 4 Arrange the following in decreasing order of reactivity towards EAS (electrophilic aromatic substitution) 18. CH3 CD3 CT3 (e) 8 conc. H2SO4 + HNO3 NO2 (a) NO2 (a) (b) (c) Sulphonation is most favourable at the carbon number... . NH (b) (a) a > b > c (d) (c) (b) c > b > a (c) a > c > b (d) c > a > b NO2 NO2 9. The major product formed in the reaction is : O Conc. HNO /conc. H SO 3 2 4 ¾ ¾¾¾¾¾¾¾¾ ¾ ® C–O O (b) O2N (c) b > a > c > d O O (d) C–O 25. C–O NO2 (d) b > c > a > d In which of the following compound electrophilic aromatic substitution take place in phenyl ring present in left hand side ? O O NO2 10. (a) C—O (c) CH2 — O O O O O (i) O O O (ii) O H SO 2 4 + A ¾ ¾¾ ¾ ® 26. (iii) (d) CH2 — C ; Reactant (A) is : O (a) iii < ii< i (b) ii < iii < i (c) i < iii < ii (d) i < ii < iii | O (a) O (b) Increasing order of rate of reaction with Br 2 AlCl 3 is : OH O OH 27. OH O (i) (ii) (a) iii < i < ii < iv (c) ii < iv < iii < i (a) (b) CH2 - C O O H3CO (i) (a) i < ii < iii < iv (ii) (b) iv < ii < i < iii (iii) (c) ii < iv < iii < i O AlCl3 O What is the major product of above Friedel-Craft reaction ? O O CO2H O2N CH3O (d) CH2 - CH - C Increasing order of equilibrium constant for the formation of a hydrate is : O (c) O (d) iv < ii < iii < 29. O (d) (iv) CO2H CO2H Which of the following compounds is the slowest to react with nitrosonium ion (NO + ) ? CH3 O OMe (iv) (iii) (b) iv < ii < i < iii i O (c) | O 12. C — NH O O O O 11. (b) Increasing order of rate of reaction with HNO 3 H 2 SO 4 is : O (d) (c) (b) C–O O2 N (c) (a) Decreasing order of rate of electrophilic aromatic substitution is : a>b>c>d a>c>b>d O C–O (a) 19. (mononitration) (a) (d) iv < ii < iii < i (b) CO2H O O CO2H (c) (d) CO2H 30. O–H What combination of acid chloride or anhydride and arene would you choose to prepare given compound ? HNO3/H2SO4 52. (A) + (more volatile) Product (A) of the above reaction is : C — CH2 — CH2 — CO2H AlCl3 + Cl — C — CH2 — CH — C — Cl (a) + (c) + AlCl3 O O NO2 NH2 NH2 (a) C (b) O NO2 , van’t-Hoff factor (i) for this reaction is : (b) 3 (c) 4 (d) 5 AlCl + Which position will be attacked most rapidly by the nitronium ion ( – NO 2 ) compound undergoes nitration with HNO 3 H 2 SO 4 : C I NO2 3 64. Ph - NO 2 + Et - Cl ¾ ¾¾ ® ( A), Product (A) of the given reaction is : NO2 (d) A = NH 2 — NH 2 HO - ,D , B = CH 3 — CH 2 — Cl, AlCl 3 B HNO3 H2SO4 (a) 2 (c) A = CH 3 — CH 2 — Cl, AlCl 3 , B = Zn(Hg), HCl A (d) NO2 NO2 I (c) I I NO2 54. NH2 I I I CH3 O || (a) A = CH 3 — C — Cl, AlCl 3 , B = Zn(Hg), HCl O || (b) A = Zn(Hg),HCl, B = CH 3 — C — Cl, AlCl 3 O NH2 I (B) 34. + 2HCl; Major product ( x) in this reaction is : x (56 – 63%) In the given conversion best yield will obtained with : O (A) (d) NO2 2ICl 53. AlCl3 + (d) (c) NH2 O O 31. (b) NO2 O O (b) (a) AlCl3 O CH3 NO2 NO2 O O OH OH OH O O ( B) (less volatile) when the (a) Ph – NH – Et D (b) no–reaction (c) (d) Et OCH3 (a) A 36. (b) B 39. (b) 65. In nitration of benzene by mixed acid the rate of reaction will be : (a) C 6 H 6 = C 6 D 6 = C 6 T6 (b) C 6 H 6 > C 6 D 6 > C 6 T6 (c) C 6 H 6 = C 6 D 6 > C 6 T6 (d) C 6 H 6 < C 6 D 6 < C 6 T6 (d) D (c) (d) 67. Which of the following ring compounds obeys Huckel's rule ? (a) C 4 H -41 (b) C 4 H +41 (c) C 4 H -2 4 What is the correct order of o p ratio when E + attacks the following system ? PhCl PhBr PhI (a) A < B < C < D (b) A = B = C = D (c) D < C < B < A (d) D < B < A < C B C D HNO3 Ph Ph Br Ph (a) Di-nitration (B) Ph Ph (b) +2 Ph Ph 73. For the reaction ; (b) A = 3 , B = 6 , C = 6 (d) A = 3 , B = 4 , C = 6 –1 Ph Ph Ph Ph 3 3 ; the best combination of reactants is : NO2 (a) C 6 H 5 Br + HNO 3 , H 2 SO 4 (b) (c) C 6 H 5 NO 2 + Br 2 , FeBr 3 (d) 74. The action of AlCl 3 in Friedel Craft’s reaction is: (a) to absorb HCl (b) (c) to produce electrophile (d) Nitration takes place at the which position of the given compound ? CMe3 D ? ¾¾® Ph Ph (d) +1 Br (a) A = 3 , B = 6 , C = 8 (c) A = 3 , B = 6 , C = 10 Ph Ph (c) –2 Ph Ph Tri-nitration (C) 41. 2AgBF 4 ¾ ¾¾¾ ® ( x) 2BF4- ; compound (x) will be : Ph HNO 3 H2 S O (80°) 4 H2SO4 (110°) Ph 69. Mono-nitration (A) O3 HN SO 4 H 2 °) (40 Br Ph How many products are capable of beings formed from toluene in each of following reaction ? CH3 (d) C 4 H 4 68. Nitration of which of the following reactant gives maximum % of meta product (using HNO 3 H 2 SO 4 ) ? (a) Toluene (b) Aniline (c) Benzene (d) Isopropyl benzene PhF A 40. Et (c) C All the hydrocarbons shown are very weak acids. One, however, is far more acidic than the others. Which one is the strongest acid ? (a) NO2 A C 6 H 5 Br + H 2 SO 4 , heat C 6 H 5 NO 2 + HBr to release HCl to produce nucleophile C CHMe2 B (a) A (c) C 43. 88. Which of the following molecules is expected to have the greatest resonance stabilization ? (b) B (d) D O Cl – C Cl (a) (A) ; Unknown (A) is : AlCl3 (b) C=O | Cl || (c) C=O (d) Ph -C - Ph 46. + AlCl3 Cl (a) H2O (A) HO– , D (b) CH2 – OH Br OH N (c) HF + 47. D (a) Suitable product of this reaction is : (b) (c) O O (d) (d) CH2CH3 (d) O CH3 — C — Cl 112. R R Br N Br CH2CH3 R N (b) (B), Product (B) in this reaction is : (c) Product. O N (a) NH2 – NH2 Fe O Br O (d) Br2(1 mole) N Cl Cl (c) 89. In the reaction given below, the major product formed is : O O C=O (a) (b) AlCl3 NH2 — NH2, NaOH (A) (80%) triethylene glycol. heat (B) (73%) CH2CH3 Product ( B ) is : NO2 HNO3 114. + H2SO4 (ortho) NO2 In the above reaction o/p ratio will be highest when : (a) R = - CH 3 (c) R = - CHMe 2 (b) R = - CH 2 - CH 3 (d) R = - CMe 3 (a) (b) (c) (d) THE FRIEDEL CRAFTS ALKYLATION CONTENTS Exercise-I Exercise-II Exercise-III Exercise-IV Exercise-V Exercise-VI Exercise-VII Answer Key : 22 :23-24 : 25-27 : 28-29 : 30-31 : 32 -37 : 38-44 : 45-61 FRIEDEL-CRAFTS REACTION THE FRIEDEL–CRAFTS ALKYLATION • Carbocations are perhaps the most important electrophiles capable of substituting on aromatic rings, because this substitution forms a new carbon–carbon bond. • Reactions of carbocations with aromatic compounds were first studied in 1877 by the French alkaloid chemist Charles Friedel and his American partner, James Crafts. • In the presence of Lewis acid catalysts such as aluminum chloride (AlCl 3) or ferric chloride (FeCl3), alkyl halides were found to alkylate benzene to give alkylbenzenes. This useful reaction is called the Friedel– Crafts alkylation. • For example, aluminum chloride catalyzes the alkylation of benzene by tert-butyl chloride. HCl gas is evolved. • This alkylation is a typical electrophilic aromatic substitution, with the tert-butyl cation acting as the electrophile. Mechanism : • Friedel–Crafts alkylation is an electrophilic aromatic substitution in which an alkyl cation acts as the electrophile. Step 1: Formation of a carbocation 3 3 + 3 3 3 Step 2: Electrophilic attack forms a sigma complex. 3 Step 3: Loss of a proton regenerates the aromatic ring and gives the alkylated product. Example : When benzene reacts with 1-chlorobutane, 60%–80% of the product (the actual percentage depends on the reaction conditions) has the rearranged alkyl substituent. (See the box on “Incipient Primary Carbocations”). Example : When benzene reacts with 1-chloro-2,2-dimethylpropane, the initially formed primary carbocation rearranges to a tertiary carbocation; in this case, 100% of the product (under all reaction conditions) has the rearranged alkyl substituent. rearrangement of the carbocation Incipient Primary Carbocations : For simplicity, the two reactions just shown that involve carbocation rearrangements were written showing the formation of a primary carbocation. However, we know that primary carbocations are too unstable to be formed. Not surprisingly, a true primary carbocation is never formed in a Friedel–Crafts alkylation. Instead, the carbocation remains complexed with the Lewis acid—a so-called “incipient” carbocation. A carbocation rearrangement occurs because the incipient carbocation has sufficient carbocation character to permit the rearrangement. Example : Use of a mixture of an alkene and an acid: Example : A mixture of an alcohol and an acid may also be used: PRACTICE – 1 1. Write the mechanism and product of the reaction given below : 2. For each reaction, show the generation of the electrophile and predict the products. (A) benzene + cyclohexene + HF (B) tert-butyl alcohol + benzene + BF3 (C) tert-butylbenzene + 2-methylpropene + HF (D) propan-2-ol + toluene + BF3 IMPORTANT POINTS : ® Friedel–Crafts alkylations are used with a wide variety of primary, secondary, and tertiary alkyl halides. With secondary and tertiary halides, the reacting electrophile is probably the carbocation. ® With primary alkyl halides, the free primary carbocation is too unstable. The actual electrophile is likely a complex of aluminum chloride with the alkyl halide. In this complex, the carbon–halogen bond is weakened (as indicated by dashed lines) and there is considerable positive charge on the carbon atom. The mechanism for the aluminum chloride-catalyzed reaction of ethyl chloride with benzene is as follows: d+ d- ¾¾ ® CH - CH L ClL AlCl CH3–CH2–Cl + AlCl3 ¬¾ ¾ 3 2 3 ® Alkenes are protonated by HF to give carbocations. Fluoride ion is a weak nucleophile and does not immediately attack the carbocation. If benzene (or an activated benzene derivative) is present, electrophilic substitution occurs. The protonation step follows Markovnikov’s rule, forming the more stable carbocation, which alkylates the aromatic ring. ® Alcohols are another source of carbocations for Friedel–Crafts alkylations. Alcohols commonly form carbocations when treated with Lewis acids such as boron trifluoride (BF3). If benzene (or an activated benzene derivative) is present, substitution may occur. Formation of the cation : Electrophilic substitution on benzene : The BF3 used in this reaction is consumed and not regenerated. A full equivalent of the Lewis acid is needed, so we say that the reaction is promoted by BF3 rather than catalyzed by BF3. LIMITATIONS OF THE FRIEDEL–CRAFTS ALKYLATION : ® Although the Friedel–Crafts alkylation looks good in principle, it has three major limitations that severely restrict its use. Limitation–1 : No reaction occurs if the substrate has either an electron-withdrawing substituent or an amino group, which reacts with the AlCl3 catalyst in an acid-base reaction. + where Y = – NR3 , –NO2, –CN, –SO3H, –CHO, –COCH3, –COOH, – COOCH3 (–NH2, –NHR, –NR2) Example : PRACTICE – 2 A. Identify the product formed during the reaction : 1. 2. 3. 4. PRACTICE – 3 1. What would be the major product of a Friedel–Crafts alkylation using the following alkyl chlorides? a. CH3CH2Cl b. CH3CH2CH2Cl c. CH3CH2CH(Cl)CH3 d. (CH3)3CCl e. (CH3)2CHCH2Cl f. CH2 CHCH2Cl PROBLEM : Derive a synthesis of p-nitro-tert-butylbenzene from benzene. SOLUTION : To make p-nitro-tert-butylbenzene, we would first use a Friedel–Crafts reaction to make tertbutylbenzene. Nitration gives the correct product. If we were to make nitrobenzene first, the Friedel–Crafts reaction to add the tert-butyl group would fail. Good Bad Limitation–2 : Like other carbocation reactions, the Friedel–Crafts alkylation is susceptible to carbocation rearrangements. As a result, only certain alkylbenzenes can be made using the Friedel–Crafts alkylation. tert-Butylbenzene, isopropylbenzene, and ethylbenzene can be synthesized using the Friedel–Crafts alkylation because the corresponding cations are not prone to rearrangement. Consider what happens, however, when we try to make n-propylbenzene by the Friedel–Crafts alkylation. Ionization with rearrangement gives isopropyl cation ¾¾ ® CH3–CH2–CH2–Cl + AlCl3 ¬¾ ¾ Reaction with benzene gives isopropylbenzene PRACTICE – 4 1. 2. 3. Limitation – 3 : ® Because alkyl groups are activating substituents, the product of the Friedel–Crafts alkylation is more reactive than the starting material. Multiple alkylations are hard to avoid. As some ethylbenzene is formed, however, it is activated, reacting even faster than benzene itself. The product is a mixture of some (ortho and para) diethylbenzenes, some triethylbenzenes, a small amount of ethylbenzene, and some leftover benzene. ® The problem of overalkylation can be minimized by using a large excess of benzene. For example, if 1 mole of ethyl chloride is used with 50 moles of benzene, the concentration of ethylbenzene is always low, and the electrophile is more likely to react with benzene than with ethylbenzene. Distillation separates the product from excess benzene. This is a common industrial approach, since a continuous distillation can recycle the unreacted benzene. Note : Lewis acid catalyst can also effect dealkylation i.e. reaction is reversible. Thus ethylbenzene with BF 3 & HF is found to be disproportionate. Limitations – 4 : Only alkyl halides can be used. Alkyl fluorides, chlorides, bromides and iodides all react well, but aryl halides and vinylic halides do not react. Aryl and vinylic carbocations are too high in energy to form under Friedel-Crafts conditions. EXAMPLE : Draw a mechanism for the following reaction, which involves two consecutive Friedel-Crafts alkylations. When drawing the mechanism, do not try to draw the two alkylations as occurring simultaneously (such a mechanism would have too many curved arrows and too many simultaneous charges). First draw the steps that install one alkyl group and then draw the steps that install the second alkyl group. MECHANISM : An interaction between a lone pair (on one of the chlorine atoms) and the Lewis acid generates a complex that can lose a leaving group (AlCl4¯) to give a tertiary carbocation. This carbocation then functions as an electrophile in an electrophilic aromatic substitution reaction. That is, benzene attacks the carbocation to give an intermediate sigma complex, which then loses a proton to restore aromaticity. All of the steps above are then repeated once again to close the ring, except this time, the electrophilic aromatic substitution reaction occurs in an intramolecular fashion. A lone pair on the chlorine atom interacts with the Lewis acid to generate a complex that can lose a leaving group (AlCl4¯) to give a tertiary carbocation. This carbocation then functions as an electrophile in an intramolecular electrophilic aromatic substitution reaction. That is, the aromatic ring attacks the carbocation to give an intermediate sigma complex, which then loses a proton to restore aromaticity: EXAMPLE : A Friedel-Crafts alkylation is an electrophilic aromatic substitution in which the electrophile (E +) is a carbocation. In previous chapters, we have seen other methods of forming carbocations, such as protonation of an alkene using a strong acid. The resulting carbocation can also be attacked by a benzene ring, resulting in alkylation of the aromatic ring. With this in mind, draw a mechanism for the following transformation: MECHANISM : Sulfuric acid is used as an aqueous solution, so we must indicate H3O+ as the proton source in our mechanism, rather than H2SO4 (because H2SO4 is a stronger acid than H3O+, so mixing H2SO4 and water causes the protons to be transferred from H2SO4 to H2O, giving H3O+). In these acidic conditions, the alkene is protonated to give a carbocation. This carbocation then serves as an electrophile in an electrophilic aromatic substitution reaction. The aromatic ring attacks the electrophile to give an intermediate sigma complex, which is then deprotonated to restore aromaticity, thereby giving the product. Water is the likely base for the final deprotonation step, since water is present in a solution of sulfuric acid (and there are no strong bases present in strongly acidic conditions). PRACTICE – 5 Predict the expected product(s) when benzene is treated with each of the following alkyl halides in the presence of AlCl3. In each case, assume conditions have been controlled to favor monoalkylation. WORKSHEET-1 Þ Identify product : 1. C6H6 + C6H5CH2Cl 2. 3C6H6 + CHCl3 3. AlCl3 ¾¾¾ ® D AlCl3 ¾¾¾ ® D AlCl3 ¾¾¾ ® D 4. + ClCH2CH2Cl + 5. 6. 7. 8. 9. D AlCl3 ¾¾¾ ® D AlCl3 ¾¾¾ ® D THE FRIEDEL–CRAFTS ACYLATION Ø An acyl group is a carbonyl group with an alkyl group attached. Acyl groups are named systematically by dropping the final -e from the alkane name and adding the -oyl suffix. Historical names are often used for the formyl group, the acetyl group, and the propionyl group, however. Ø An acyl chloride is an acyl group bonded to a chlorine atom. Acyl chlorides are made by reaction of the corresponding carboxylic acids with thionyl chloride. Therefore, acyl chlorides are also called acid chlorides. Ø In the presence of aluminum chloride, an acyl chloride reacts with benzene (or an activated benzene derivative) to give a phenyl ketone: an acylbenzene. The Friedel–Crafts acylation is analogous to the Friedel–Crafts alkylation, except that the reagent is an acyl chloride instead of an alkyl halide and the product is an acylbenzene (a “phenone”) instead of an alkylbenzene. Friedel–Crafts acylation Example : Mechanism of Acylation • The mechanism of Friedel–Crafts acylation (shown next) resembles that for alkylation, except that the electrophile is a resonance-stabilized acylium ion. The acylium ion reacts with benzene or an activated benzene derivative via an electrophilic aromatic substitution to form an acylbenzene. • Friedel–Crafts acylation is an electrophilic aromatic substitution with an acylium ion acting as the electrophile. Step 1: Formation of an acylium ion. Steps 2 and 3: Electrophilic attack forms a sigma complex, and loss of a proton regenerates the aromatic system. Step 4: Complexation of the product. The product complex must be hydrolyzed (by water) to release the free acylbenzene. • The product of acylation (the acylbenzene) is a ketone. The ketone’s carbonyl group has nonbonding electrons that complex with the Lewis acid (AlCl3), requiring a full equivalent of AlCl3 in the acylation. The initial product is the aluminum chloride complex of the acylbenzene. Addition of water hydrolyzes this complex, giving the free acylbenzene. EXAMPLES : 1. MECHANISM : The anhydride interacts with the Lewis acid to give a complex which loses a leaving group to produce an acylium ion. This acylium ion then serves as an electrophile in an electrophilic aromatic substitution reaction. The aromatic ring attacks the electrophile to give an intermediate sigma complex, which is then deprotonated to restore aromaticity, thereby giving the product. An acetate ion is the likely base for the final deprotonation step, since acetate is a by-product of the reaction. Acetate is formed when the leaving group (of the second step of mechanism) breaks apart into AICI3 and acetate (as shown). 2. 3. 4. 5. WORKSHEET–2 Na -Hg AlCl3 B ¾¾¾ ® A ¾¾¾® HCl 1. AlCl 2. 3 ® C6H6 + RCOCl ¾¾¾¾ PhNO 3. 3 ® C6H6 + C6H5CH2COCl ¾¾¾ CS 4. 5. 2 AlCl 2 CH3COCl ¾¾¾¾ ® AlCl3 AlCl3 ¾¾¾ ® 6. 7. 8. Find out the final product in the given reactions : Note : + 1. The electrophile in the Friedel–Crafts acylation appears to be a large, bulky complex, such as R–C O¯AlCl4. Para substitution usually prevails when the aromatic substrate has an ortho, para-directing group, possibly because the electrophile is too bulky for effective attack at the ortho position. For example, when ethylbenzene reacts with acetyl chloride, the major product is p-ethylacetophenone. 2. One of the most attractive features of the Friedel–Crafts acylation is the deactivation of the product toward further substitution. The acylbenzene has a carbonyl group (a deactivating group) bonded to the aromatic ring. Since Friedel–Crafts reactions do not occur on strongly deactivated rings, the acylation stops after one substitution. 3. Friedel–Crafts acylation overcomes two of the three limitations of the alkylation: (a) The acylium ion is resonance-stabilized, so that no rearrangements occur; (b) the acylbenzene product is deactivated, so that no further reaction occurs. Like the alkylation, however, the acylation fails with strongly deactivated aromatic rings. SYNTHESIS OF IBUPROFEN : There are several approaches to the synthesis of the analgesic anti-inflammatory drug ibuprofen. Here is one that employs a relatively simple sequence of reactions, beginning with a Friedel–Crafts acylation of isobutylbenzene. The alkyl substituent is weakly electron releasing, and thus activates the ring towards electrophilic substitution. It also directs further substitution to the ortho and para positions. As in most Friedel–Crafts acylations, the para product predominates strongly over the ortho, a consequence of the relatively large size of the electrophilic reagent (see Section 8.4.3). In this case, we also have a quite large alkyl substituent, again disfavouring the ortho product. The subsequent steps are relatively straightforward. Sodium borohydride reduction of the ketone gives an alcohol (see Section 7.5), then the alcohol is converted into a nitrile by successive nucleophilic substitution reactions. Note that a two-stage process is involved. Since hydroxide is a poor leaving group, nucleophilic substitution requires acidic conditions to protonate the hydroxyl to provide a better leaving group. We can formulate an SN1 conversion, since this would involve a favourable benzylic carbocation. HCN is a weak acid (pKa 9.1), so it is not very effective in protonating the hydroxyl group. Thus, the two-stage process is used, with displacement of hydroxyl via bromide, then subsequent displacement of bromide by cyanide, the latter step usually being an SN2 process. Lastly, the nitrile group is hydrolysed to a carboxylic acid. The starting material, isobutylbenzene, is readily available, but could be synthesized by exploiting another Friedel–Crafts reaction. SUMMARY COMPARISON OF FRIEDEL–CRAFTS ALKYLATION AND ACYLATION Alkylation Acylation The alkylation cannot be used with strongly Also true: Only benzene, halobenzenes, deactivated and activated derivatives are suitable. The carbocations involved in the alkylation may Resonance-stabilized acylium ions are not rearrange. prone to rearrangement. Polyalkylation is commonly a problem. The acylation forms a deactivated acylbenzene, which does not react further. Summary of Directing and Activating or Deactivating Effects of Some Common Functional Groups (Groups are listed in decreasing order of activation) SOME IMPORTANT POINTS ® Carboxylic acid anhydrides, compounds of the type , can also serve as sources of acyl cations and, in the presence of aluminum chloride, acylate benzene. One acyl unit of an acid anhydride becomes attached to the benzene ring, while the other becomes part of a carboxylic acid. ® Succinic anhydride, the structure of which is shown, is a cyclic anhydride often used in Friedel-Crafts acylations. Give the structure of the product obtained when benzene is acylated with succinic anhydride in the presence of aluminum chloride. ® An important difference between Friedel-Crafts alkylations and acylations is that acyl cations do not rearrange. The acyl group of the acyl chloride or acid anhydride is transferred to the benzene ring unchanged. The reason for this is that an acyl cation is so strongly stabilized by resonance that it is more stable than any ion that could conceivably arise from it by a hydride or alkyl group shift. ® Polyacylation is not observed, because introduction of an acyl group deactivates the ring toward further acylation. This will be explained in more detail in the upcoming sections. PROBLEM : The following compound cannot be made with either a Friedel-Crafts alkylation or acylation. Explain. PROBLEM : Identify whether each of the following compounds can be made using a direct Friedel-Crafts alkylation or whether it is necessary to perform an acylation followed by a Clemmensen reduction to avoid carbocation rearrangements: (A) (B) (C) (D) EXERCISE–1 Prepare the following by friedal craft reaction : Reactant 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. EXERCISE–2 1. What product is formed when benzene is treated with each organic halide in the presence of AlCl3? (A) (CH3)2CHCl 2. (B) (C) What acid chloride would be needed to prepare each of the following ketones from benzene using a FriedelCrafts acylation ? (A) 3. (B) Which halides are unreactive in a Friedel-Crafts alkylation reaction ? (A) 4. (B) (C) (D) Draw the product of each reaction. (A) + H2SO 4 ¾¾ ¾¾® (C) 5. (C) H SO (B) 4® ¾¾2 ¾¾ (D) 4® ¾¾2 ¾¾ H SO Draw the products formed when each compound is treated with HNO3 and H2SO4. (A) (B) (C) (D) 6. Draw the products of each reaction. (A) (B) CH3CH2Cl ¾¾ ¾ ¾¾® (C) AlCl3 (E) H2SO 4 (D) (F) (G) (H) 7. Write out two different routes to ketone A using a Friedel-Crafts acylation. 8. Draw the products of each reaction. HNO3 ¾¾ ¾ ¾® (A) H2SO 4 (C) HNO3 ¾¾ ¾ ¾® (B) (D) H2SO 4 (E) HNO 3® ¾¾ ¾ ¾ HNO 3 ¾¾ ¾ ¾ ® H2SO 4 (F) Br2 ¾¾ ¾® hv Br2 ¾¾ ¾® FeBr3 Br2 ¾¾ ¾® FeBr3 EXERCISE–3 1. Which of the following alkyl halides would you expect to undergo Friedel–Crafts reaction without rearrangement? Explain. (A) CH3CH2Cl (B) CH3CH2CH(Cl)CH3 (C) CH3CH2CH2Cl (D) (CH3)3CCH2Cl (E) Chlorocyclohexane 2. What is the major monosubstitution product from the Friedel–Crafts reaction of benzene with 1-chloro-2methylpropane in the presence of AlCl3? 3. Identify the carboxylic acid chloride that might be used in a Friedel-Crafts acylation reaction to prepare each of the following acylbenzenes : (A) 4. (B) Propose products (if any) and mechanisms for the following reactions: (A) chlorocyclohexane with benzene (B) methyl chloride with anisole (C) 3-chloro-2,2-dimethylbutane with isopropylbenzene 5. Predict the products (if any) of the following reactions. (A) (excess) benzene + isobutyl chloride + AlCl3 (B) (excess) toluene + butan-1-ol + BF3 (C) (excess) nitrobenzene + 2-chloropropane + AlCl3 (D) (excess) benzene + 3,3-dimethylbut-1-ene + HF 6. Complete the following reactions : (i) (ii) (iii) (iv) (v) (vi) (vii) (viii) What is the relationship between the products (A) & (B) : (A) Identical (B) Enantiomers (C) Diasteromers (D) Meso 7. Would you expect the Friedel-Craft reaction of benzene with (R)-2-chlorobutane to yield optically active or racemic product ? Explain. 8. 9. Rank the following aromatic compounds in the expected order of their reactivity toward Friedel-Crafts alkylation. Which compound are unreactive ? (A) Bromobenzene (B) Toluene (C) Phenol (D) Aniline (E) Nitrobenzene (F) p-Bromotoluene The carbocation electrophile in a Friedel-Crafts reaction can be generated in ways other than by reaction of an alkyl chloride with AlCl3. For example, reaction of benzene with 2-methylpropene in the presence of H3PO4 yields tert-butylbenzene. Propose a mechanism for this reaction. 10. Which reactions will produce the desired product in good yield? You may assume that aluminum chloride is added as a catalyst in each case. For the reactions that will not give a good yield of the desired product, predict the major products. Reagents 11. Desired Product (A) benzene + n-butyl bromide n-butylbenzene (B) ethylbenzene + tert-butyl chloride p-ethyl-tert-butylbenzene (C) bromobenzene + ethyl chloride p-bromoethylbenzene (D) benzamide (PhCONH2) + CH3CH2Cl p-ethylbenzamide (E) toluene + HNO3, H2SO4, heat 2,4,6-trinitrotoluene (TNT) Show how you would synthesize the following aromatic derivatives from benzene. (A) p-tert-butylnitrobenzene (B) p-toluenesulfonic acid (C) p-chlorotoluene EXERCISE–4 A. Identify the product formed during the reaction. Reactant 1. 2. 3. 4. 5. 6. 3 7. Reactant 8. 9. 10. 11. 12. 13. 14. 15. B. Compare rate for electrophilic aromatic substitution 16. (A) 17. (B) 18. (C) 19. (D) 2 20. (E) 21. (F) D. Identify reactant 22. A + B ¾® 23. AlCl3 A + B ¾¾ ¾ ¾® 24. AlCl3 A + B ¾¾ ¾ ¾® 25. AlCl3 A + B ¾¾ ¾ ¾® C. Match the column : 26. Column-I (A) AlCl3 + R–Cl ¾¾ ¾ ¾® 2 Column-II (P) Friedel craft alkylation (B) (Q) Friedel craft acylation (C) (R) Nitration (D) (S) Halogenation (T) Substitution reaction ‘‘Be motivated, be creative, be passionate’’ 3 EXERCISE–5 1. 2. 3. 4. 3 5. 3 6. 3 3 7. 8. 9. EXERCISE–6 1. (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) All of these (A) (B) (C) (D) 2. 3. 4. 5. 6. (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) 7. 8. 9. 10. (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) 11. 12. 13. 14. (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) 15. 16. 17. 18. (A) (B) (C) (D) (A) (B) (C) (D) 19. 20. (A) (B) (C) (D) No reaction 21. (A) (B) (C) (D) No reaction (A) (B) (C) (D) No reaction 22. EXERCISE–7 1. (A) o-nitro nitrobenzene (C) p-nitro nitrobenzene (B) m-nitro nitrobenzene (D) no reaction 2. (A) (B) (C) (A) (B) (C) (A) (B) (C) (D) No reaction 3. (D) 4. (D) 5. (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) 6. 7. 8. (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) 9. 10. 11. (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) 12. 13. 14. 15. (A) (B) (C) (D) (A) (B) (C) (D) (A) (B) (C) (D) 16. 17. 18. (A) (B) (C) (D) 19. (A) (B) (C) (D) (A) (B) (C) (D) 20. 21. (A) (B) (C) (D) (A) (B) (C) (D) 22.