Chapter 29: Friedel-Crafts Alkylation of Benzene
advertisement

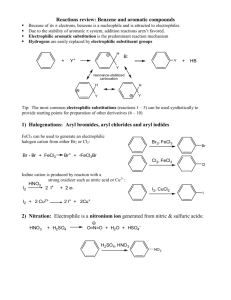
Chapter 29 Friedel-Crafts Alkylation of Benzene Purpose • In this experiment a mixture of benzene and alkyl chloride is treated with AlCl3 (Lewis acid). The dialkyl product is isolated by adding water and extracting. • The organic phase is then washed, dried and evaporated to give an oily product which should solidify upon cooling. You may also use filtering paper to absorb any oil from the solid product. Reaction Mechanism • The Friedel-Crafts alkylation is a typical electrophilic substitution reaction. The electrophile is t-butyl cation generated by the reaction of alkyl halide and Lewis acid. The dialkyl product is formed stepwise. “Mystery” Modification • Shown product can be obtained in case of using excess of t-Butyl Chloride (at least 5 mmols per 1 mmol of benzene) under the same reaction conditions. You should provide a reasonable reaction mechanism in your lab report. Comments • Follow pp. 438-440 (microscale). • Follow the lab handout for all experimental procedures. • AlCl3 is highly hydroscopic and looses catalytic activity after absorbtion moisture from the air. Keep it AWAY from prolong contact with air! • Perform GC/MS and optionally IR analysis of the products from both reactions. • Prepare samples of the products in CDCl3 for NMR. • Calculate yields. Moderate yields should be achieved. (Moles Product / Moles Starting Material) x 100 = % Safety • Benzene is highly flammable, irritating to eyes and skin and may cause lung damage if swallowed. Also, as shown on animal models, may cause cancer and inheritable genetic damages by prolonged exposure. Do not swallow, do not inhale and avoid contact with eyes and skin. Wear gloves and handle with extreme caution. • Aluminum chloride is corrosive, wear gloves and handle with care. • Again: wearing rubber gloves is absolutely necessary during this lab! You will work with toxic, carcinogenic and corrosive compounds.