CHEM 322 – Spring 2015 NAME: Problem Set 2
advertisement

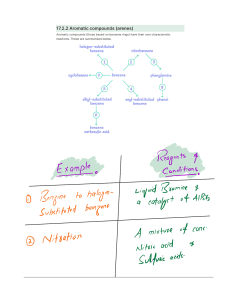
CHEM 322 – Spring 2015 Problem Set 2 Due 3/9/15 NAME: Discussion Section: 1. Explain why the trifluoromethyl group is meta-directing in electrophilic aromatic substitution. Would you expect CF3 to be activating or deactivating? Why? 2. Give the expected major product(s) of each of the following reactions. 3. Predict the products of the following transformations and provide a reasonable arrow pushing mechanism. 4. In an attempt to synthesize n-propylbenzene A from benzene, Dr. X performed a Friedel-Crafts alkylation using n-propyl bromide in the presence of AlBr3. Instead of obtaining the desired product A, B was observed as the only major product. Provide a reasonable mechanism which accounts for the formation of B. Provide an alternative route for the synthesis of the desired product A. 5. Devise a synthesis of the following molecules starting from benzene. 6. Provide a mechanism which is consistent with the following observation: 7. Provide the structures of the intermediates and final product resulting from this series of transformations. Provide a mechanism which accounts for the formation of A.