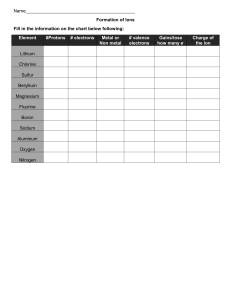
Lewis Symbols and the Octet Rule The electrons involved in chemical bonding are the valence electrons, which, for most atoms, are its outermost electrons. The American chemist, Gilbert Newton Lewis (1875–1946), suggested a simple way of showing the valence electrons in an atom and tracking them during bond formation, using what are now known as Lewis symbols. The Lewis symbol for an element consists of the element’s chemical symbol plus a dot for each valence electron. For example, sulfur has six valence electrons. Its Lewis symbol is: The dots are placed on the four sides of the chemical symbol — top, bottom, left, and right — and each side can accommodate up to two electrons. All four sides are equivalent, which means that the choice of sides for placing the electrons is arbitrary. However, it is recommended to spread out the dots as much as possible. SLP’s Notes! How do you know the valence electron of a chemical element? To make an effective Lewis symbol, one must know first the number of valence electrons in a chemical element. As such, there are two possible ways of determining the valence electron of an element: (1) Electron Configuration The electron configuration refers to the distribution of electrons in an atom. Since it reports electron distribution, you can check which electrons are the outermost. Let’s take sulfur for example. Sulfur has an electron configuration of: [Ne] 3s23p4 Here, the outermost electrons are marked in red. In a periodic table, they are usually the last two sections of the electron configuration. To proceed with the example, 2 + 4 = 6 valence electrons, in which we saw the Lewis symbol earlier. (2) Patterns in the Periodic Table If electron configuration is quite challenging, you might want to take note of patterns in the periodic table instead. Elements found in the same column generally have the same number of valence electrons. Refer to the following periodic table. The number indicated is the number of valence electrons for that group. Columns with no indicated number do not follow this pattern. 1 8 2 3 4 5 6 7 Using this pattern, we can also see that sulfur has 6 valence electrons. Thus, we can now draw its Lewis symbol. Atoms often gain, lose, or share electrons to achieve the same number of electrons as the noble gas closest to them in the periodic table. The noble gases have very stable electron arrangements, which result to lack of chemical reactivity. Because noble gases generally have eight valence electrons, many atoms undergoing reactions end up with eight valence electrons. This observation has led to a guideline known as the octet rule: Atoms tend to gain, lose, or share electrons until they are surrounded by eight valence electrons. In a Lewis symbol, an octet is shown as four pairs of valence electrons arranged around the element symbol, as follows: As such, the octet rule provides a useful framework for introducing many important concepts of bonding. Brown, T.L., LeMay, H.E., Bursten, B.E., Murphy, C.J., Woodward, P.M., & Stoltzfus, M.W. (2018). Chemistry the Central Science. 14th ed. Pearson Education Ltd.